In Vitro Diagnostics Ivd Packaging Market Report
Published Date: 01 February 2026 | Report Code: in-vitro-diagnostics-ivd-packaging
In Vitro Diagnostics Ivd Packaging Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the In Vitro Diagnostics (IVD) packaging market from 2023 to 2033, highlighting key insights, market size, forecasts, and trends shaping the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
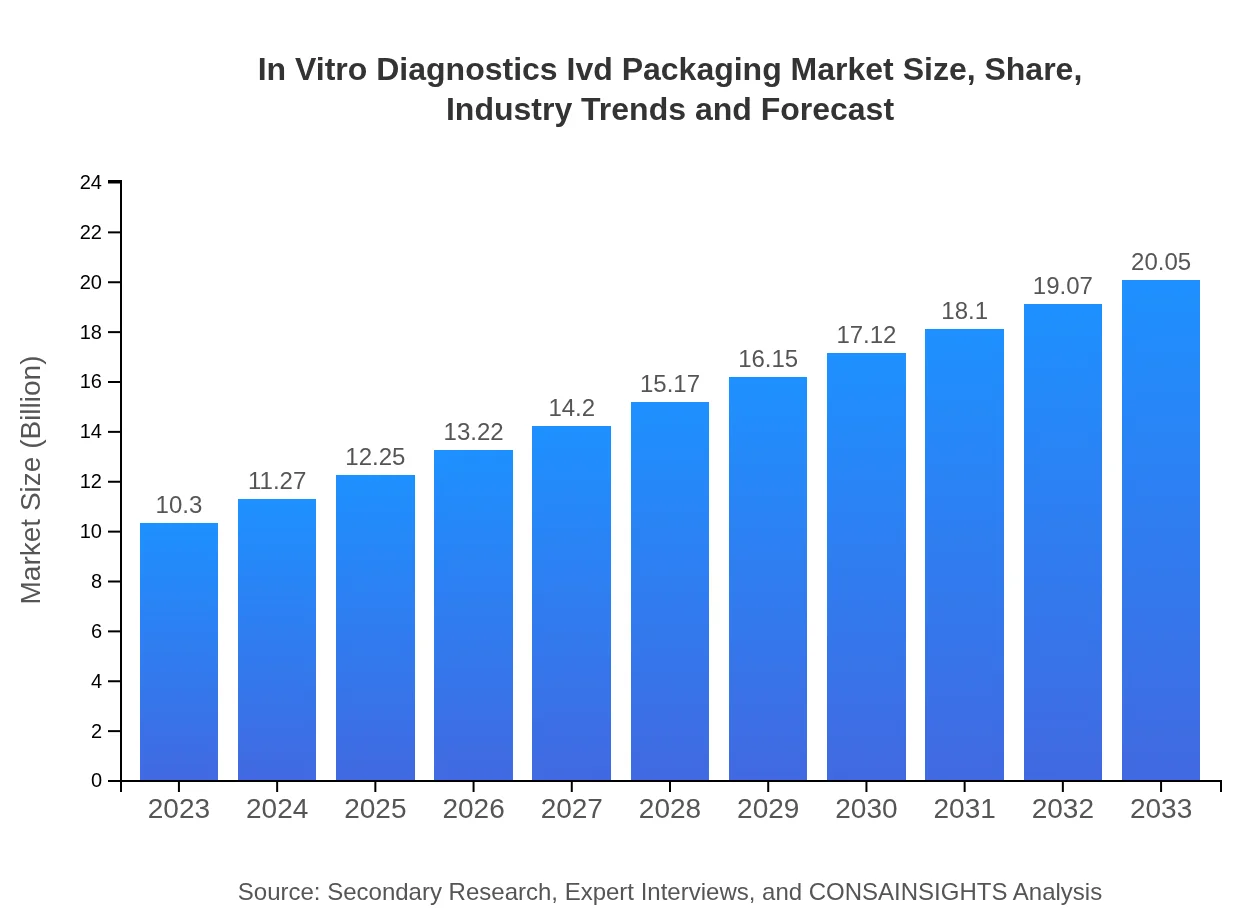
| 2023 Market Size | $10.30 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $20.05 Billion |
| Top Companies | Thermo Fisher Scientific, BD (Becton, Dickinson and Company), Roche Diagnostics, Siemens Healthineers, Abbott Laboratories |
| Last Modified Date | 01 February 2026 |
In Vitro Diagnostics Ivd Packaging Market Overview
Customize In Vitro Diagnostics Ivd Packaging Market Report market research report
- ✔ Get in-depth analysis of In Vitro Diagnostics Ivd Packaging market size, growth, and forecasts.
- ✔ Understand In Vitro Diagnostics Ivd Packaging's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in In Vitro Diagnostics Ivd Packaging
What is the Market Size & CAGR of In Vitro Diagnostics Ivd Packaging Market in 2023 and 2033?
In Vitro Diagnostics Ivd Packaging Industry Analysis
In Vitro Diagnostics Ivd Packaging Market Segmentation and Scope
Tell us your focus area and get a customized research report.
In Vitro Diagnostics Ivd Packaging Market Analysis Report by Region
Europe In Vitro Diagnostics Ivd Packaging Market Report:
Europe's market, currently valued at $3.08 billion, is projected to reach $6.00 billion by 2033. Growth is fueled by increasing demand for PCR testing and a strong regulatory framework.Asia Pacific In Vitro Diagnostics Ivd Packaging Market Report:
The Asia Pacific IVD packaging market, valued at $1.93 billion in 2023, is projected to reach $3.76 billion by 2033, driven by increasing healthcare expenditure and a focus on advanced diagnostic solutions.North America In Vitro Diagnostics Ivd Packaging Market Report:
North America dominates the market with a 2023 valuation of $3.87 billion and a projection of $7.53 billion by 2033. The region's advanced healthcare systems and technological innovation are key drivers.South America In Vitro Diagnostics Ivd Packaging Market Report:
In South America, the market is anticipated to grow from $0.77 billion in 2023 to $1.50 billion by 2033. Factors such as the rising prevalence of diseases and improved healthcare infrastructure contribute to this growth.Middle East & Africa In Vitro Diagnostics Ivd Packaging Market Report:
The Middle East and Africa region exhibits growth from $0.65 billion in 2023 to $1.26 billion by 2033, owing to increasing investments in healthcare infrastructure and rising awareness of diagnostic solutions.Tell us your focus area and get a customized research report.
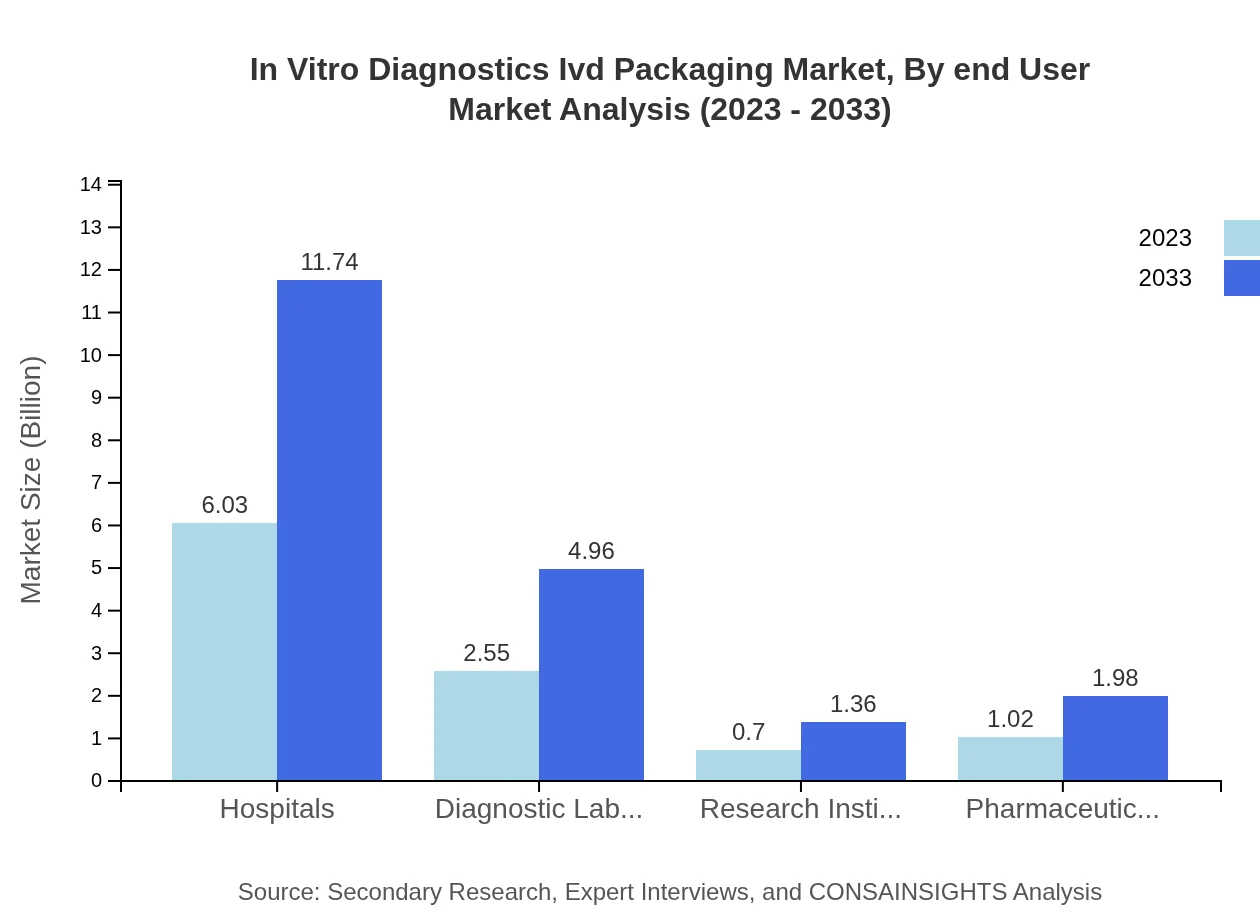
In Vitro Diagnostics Ivd Packaging Market Analysis By End User
The end-user segment shows noteworthy figures for 2023 and 2033: Hospitals: $6.03B (58.57%), Diagnostic Laboratories: $2.55B (24.76%), Research Institutes: $0.70B (6.77%), Pharmaceutical Companies: $1.02B (9.90%). By 2033, expected sizes are $11.74B, $4.96B, $1.36B, and $1.98B respectively, maintaining similar market shares.
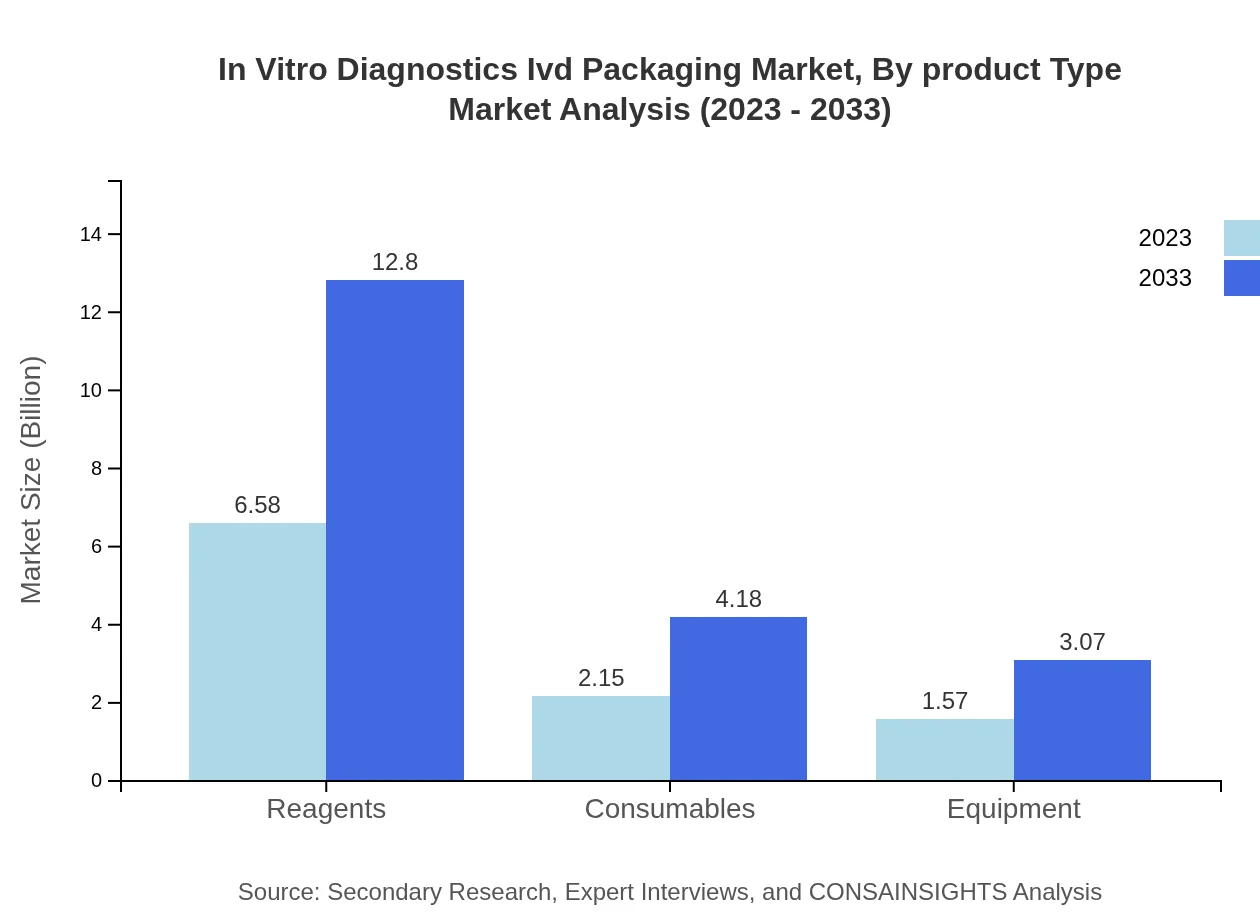
In Vitro Diagnostics Ivd Packaging Market Analysis By Product Type
Product types include: Reagents: $6.58B (63.85%) in 2023, growing to $12.80B by 2033; Consumables: $2.15B (20.86%), reaching $4.18B; Equipment: $1.57B (15.29%), expanding to $3.07B. The market trends highlight a significant focus on reagent and consumable segmentation.
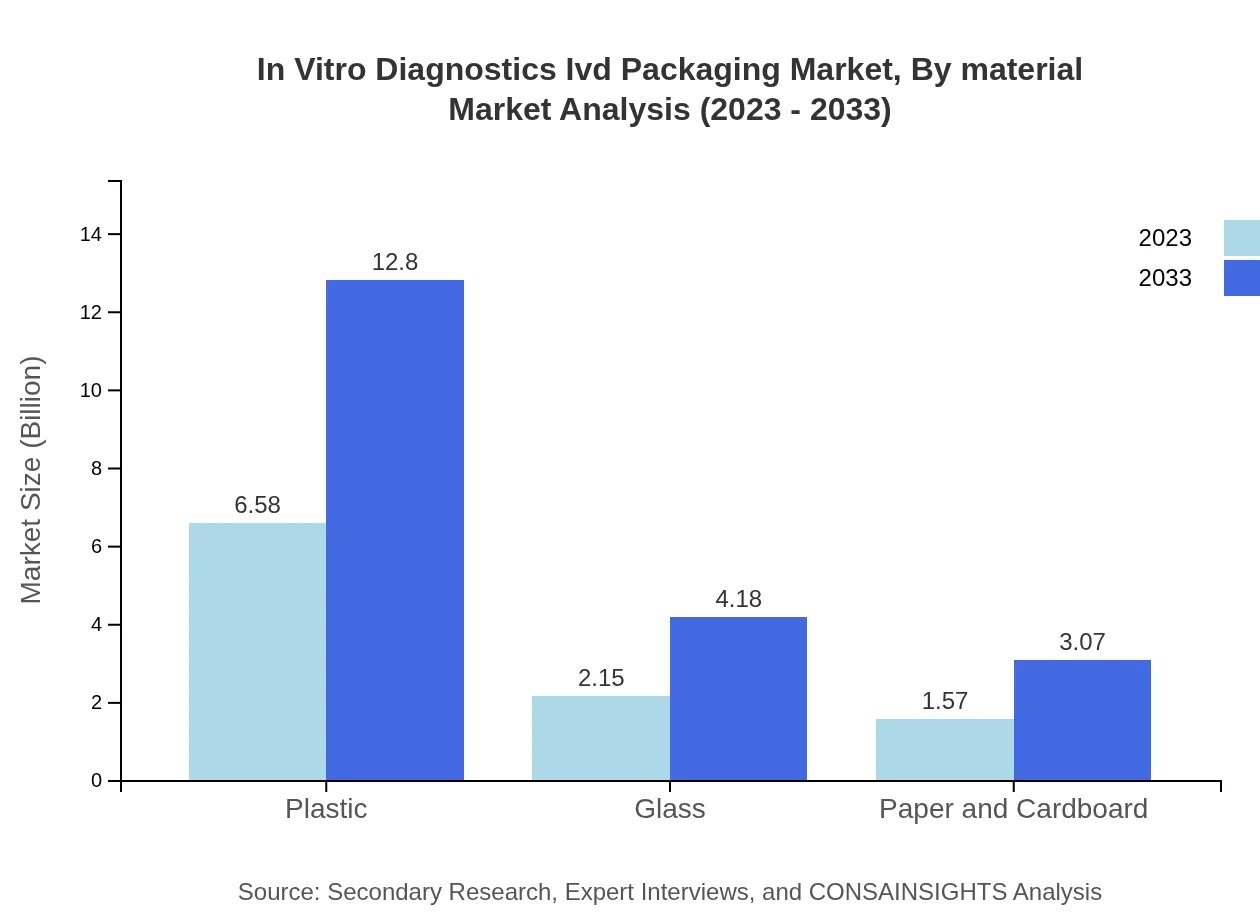
In Vitro Diagnostics Ivd Packaging Market Analysis By Material
Material segmentation shows significant distribution: Plastic: $6.58B (63.85%) in 2023 to $12.80B; Glass: $2.15B (20.86%) to $4.18B; Paper and Cardboard: $1.57B (15.29%) to $3.07B. The dominance of plastic can be attributed to its lightweight and cost-effective nature.
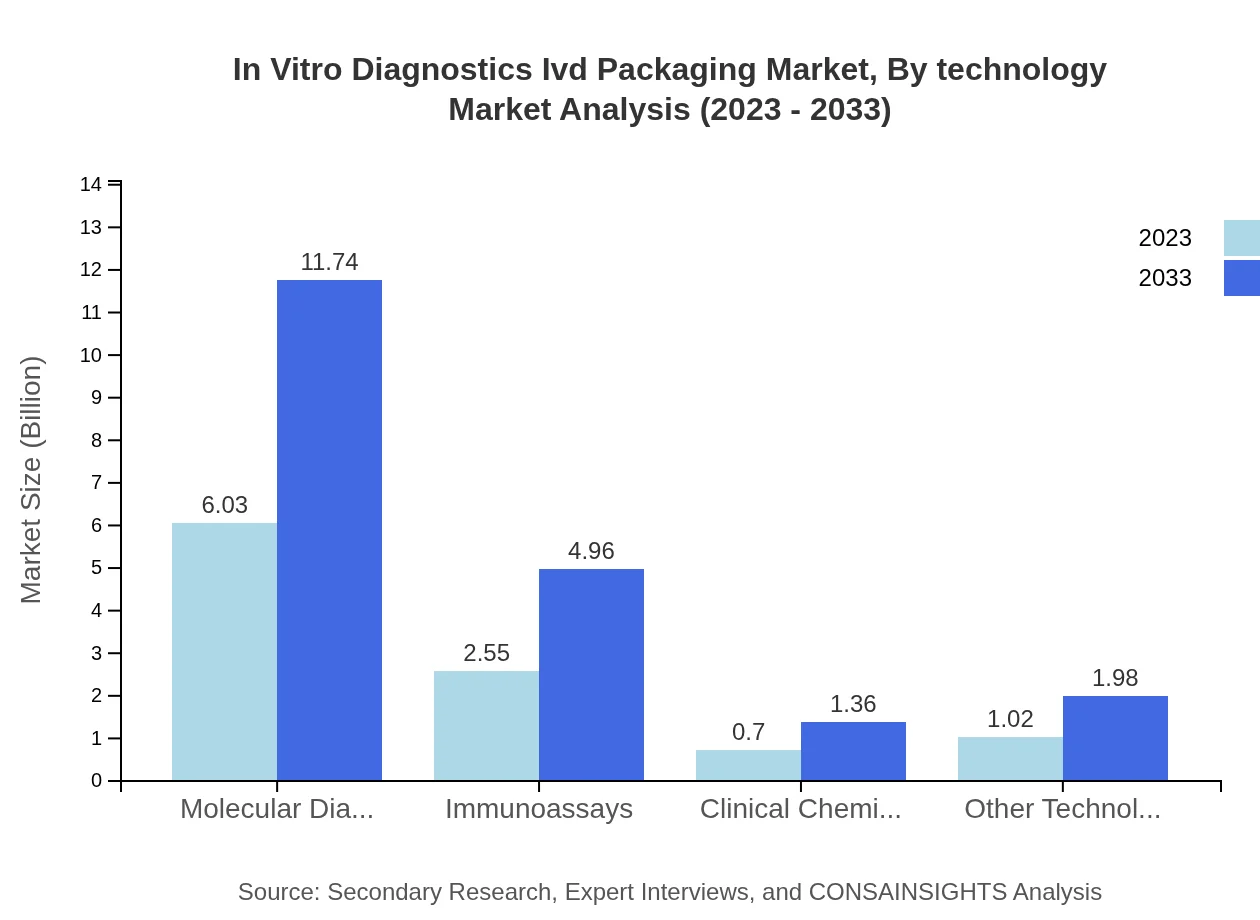
In Vitro Diagnostics Ivd Packaging Market Analysis By Technology
Technology segments show growth insights: Molecular Diagnostics: 2023 $6.03B (58.57%) to $11.74B; Immunoassays: $2.55B (24.76%) to $4.96B; Clinical Chemistry: $0.70B (6.77%) to $1.36B. The advancements in molecular diagnostics are particularly impactful in driving market growth.
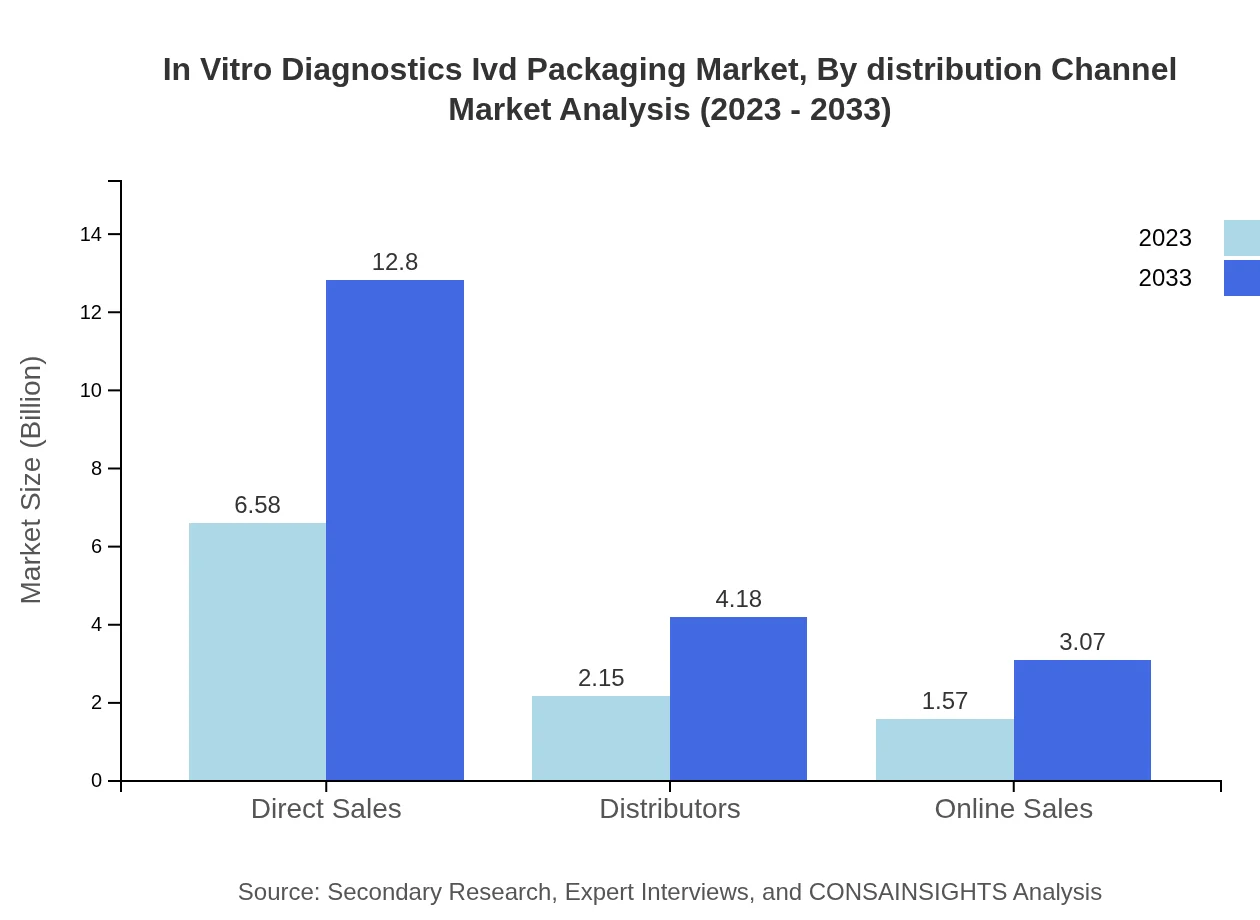
In Vitro Diagnostics Ivd Packaging Market Analysis By Distribution Channel
Distribution channels reveal trends: Direct Sales: $6.58B (63.85%), Online Sales: $1.57B (15.29%), Distributors: $2.15B (20.86%). Expect growth for direct sales and increasing online sales embracing e-commerce trends.
In Vitro Diagnostics Ivd Packaging Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in In Vitro Diagnostics Ivd Packaging Industry
Thermo Fisher Scientific:
A leader in serving science, Thermo Fisher provides a plethora of IVD solutions and packaging innovations that enhance diagnostic applications.BD (Becton, Dickinson and Company):
BD is renowned for its medical technology solutions, offering advanced IVD packaging products that ensure safety and reliability.Roche Diagnostics:
With a strong focus on innovation, Roche provides integrated IVD solutions along with sustainable and efficient packaging options.Siemens Healthineers:
Focusing on diagnostic excellence, Siemens Healthineers offers advanced IVD packaging technologies that cater to modern healthcare needs.Abbott Laboratories:
A major player in the healthcare industry, Abbott provides a comprehensive range of IVD packaging solutions that optimize product integrity.We're grateful to work with incredible clients.









FAQs
What is the market size of In Vitro Diagnostics (IVD) packaging?
The In Vitro Diagnostics (IVD) packaging market is valued at approximately $10.3 billion in 2023 and is projected to grow at a CAGR of 6.7% from 2023 to 2033.
What are the key market players or companies in the In Vitro Diagnostics (IVD) packaging industry?
Key players in the IVD packaging industry include Becton Dickinson, Thermo Fisher Scientific, 3M Company, and others, each contributing to technological advancements and innovative packaging solutions.
What are the primary factors driving the growth in the In Vitro Diagnostics (IVD) packaging industry?
Growth is primarily driven by advancements in healthcare, increasing prevalence of chronic diseases, rising demand for diagnostics, and the need for efficient packaging solutions to ensure product integrity and safety.
Which region is the fastest Growing in the In Vitro Diagnostics (IVD) packaging?
North America is the fastest-growing region, expected to reach a market size of $7.53 billion by 2033, followed by Europe and Asia Pacific, reflecting rising healthcare investments.
Does ConsaInsights provide customized market report data for the In Vitro Diagnostics (IVD) packaging industry?
Yes, ConsaInsights offers customized market reports tailored to specific needs in the IVD packaging industry, ensuring comprehensive insights and data relevant to clients' requirements.
What deliverables can I expect from this In Vitro Diagnostics (IVD) packaging market research project?
The project will deliver in-depth market analysis, growth forecasts, competitive landscape insights, segment data, and regional trends specific to the IVD packaging industry.
What are the market trends of In Vitro Diagnostics (IVD) packaging?
Current trends include a shift toward sustainable packaging, increasing automation in packaging processes, and growth in personalized medicine, influencing packaging requirements.