Medical Device Security Market Report
Published Date: 31 January 2026 | Report Code: medical-device-security
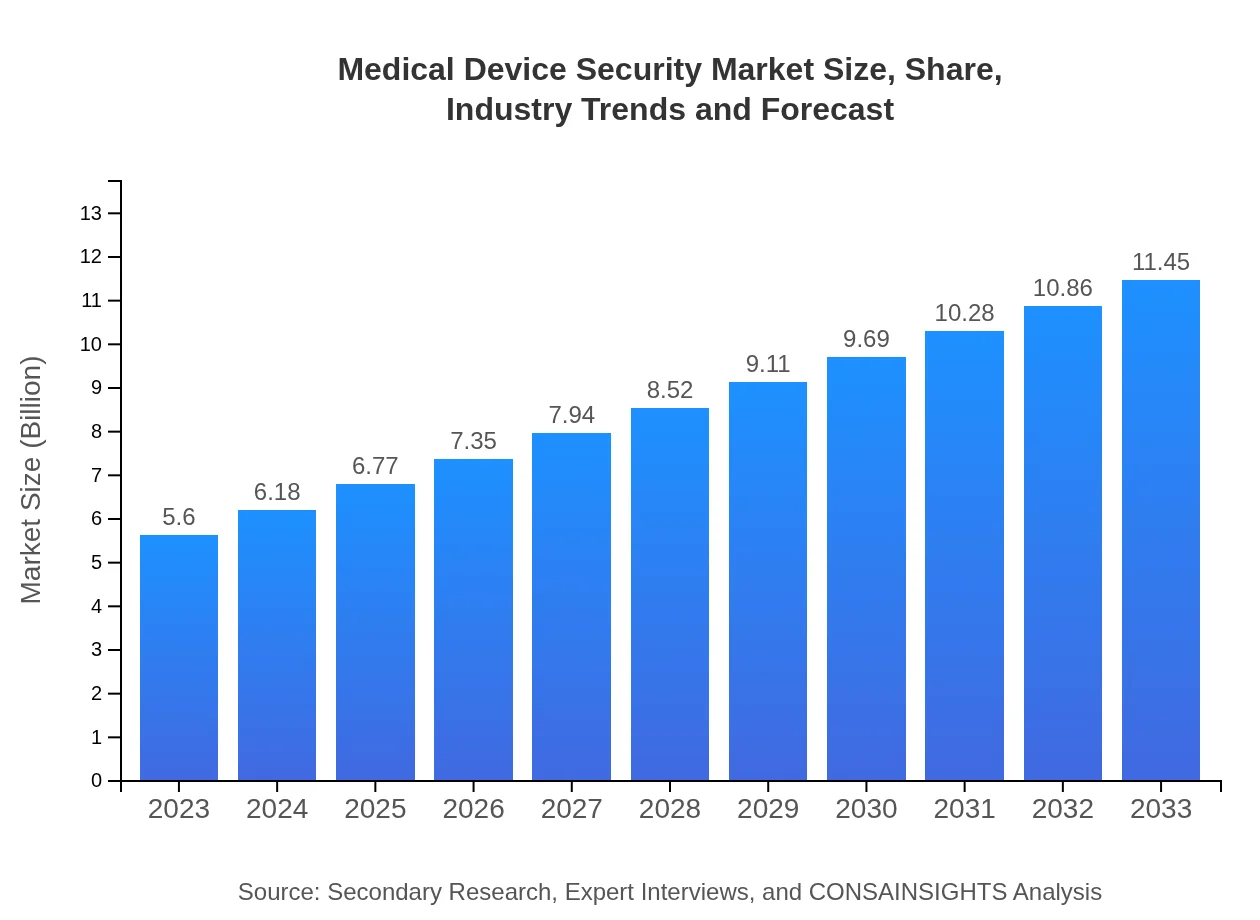
Medical Device Security Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Medical Device Security market from 2023 to 2033, highlighting market dynamics, size, growth forecasts, regional insights, and the key players impacting the industry landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $11.45 Billion |
| Top Companies | IBM Corporation, Medtronic , Palo Alto Networks, Cisco Systems, Inc., Fortinet |
| Last Modified Date | 31 January 2026 |
Medical Device Security Market Overview
Customize Medical Device Security Market Report market research report
- ✔ Get in-depth analysis of Medical Device Security market size, growth, and forecasts.
- ✔ Understand Medical Device Security's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Medical Device Security
What is the Market Size & CAGR of Medical Device Security market in 2033?
Medical Device Security Industry Analysis
Medical Device Security Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Medical Device Security Market Analysis Report by Region
Europe Medical Device Security Market Report:
In Europe, the market is estimated to grow from USD 1.79 billion in 2023 to USD 3.66 billion by 2033. The European Union's strict data protection regulations are stimulating the demand for comprehensive security solutions in healthcare settings. Additionally, the rising cyber threats within healthcare institutions necessitate stringent cybersecurity measures.Asia Pacific Medical Device Security Market Report:
In the Asia Pacific region, the Medical Device Security market is projected to grow from USD 1.08 billion in 2023 to USD 2.21 billion by 2033. This growth is fueled by increasing healthcare expenditures, a burgeoning digital health sector, and growing awareness of cybersecurity risks. Countries like China and India are ramping up investments in health IT systems, propelling the adoption of security solutions for medical devices.North America Medical Device Security Market Report:
North America is a leading region, with the market projected to surge from USD 1.87 billion in 2023 to USD 3.82 billion by 2033. High healthcare IT spending, coupled with the presence of prominent cybersecurity firms and increasing regulatory scrutiny, drives robust demand for medical device security solutions. The U.S. continues to spearhead innovations and standards in healthcare cybersecurity.South America Medical Device Security Market Report:
The South American market is expected to expand from USD 0.47 billion in 2023 to USD 0.96 billion by 2033. The region is gradually recognizing the importance of securing medical devices amid rising cyber threats. Efforts to enhance healthcare infrastructure and the introduction of supportive government policies will likely catalyze market growth.Middle East & Africa Medical Device Security Market Report:
The Middle East and Africa market is anticipated to grow from USD 0.39 billion in 2023 to USD 0.80 billion by 2033. There is a burgeoning focus on improving healthcare services within the region, and as technology adoption increases, so too does the need for robust medical device security. Government initiatives aimed at enhancing healthcare digitalization will further propel market growth.Tell us your focus area and get a customized research report.
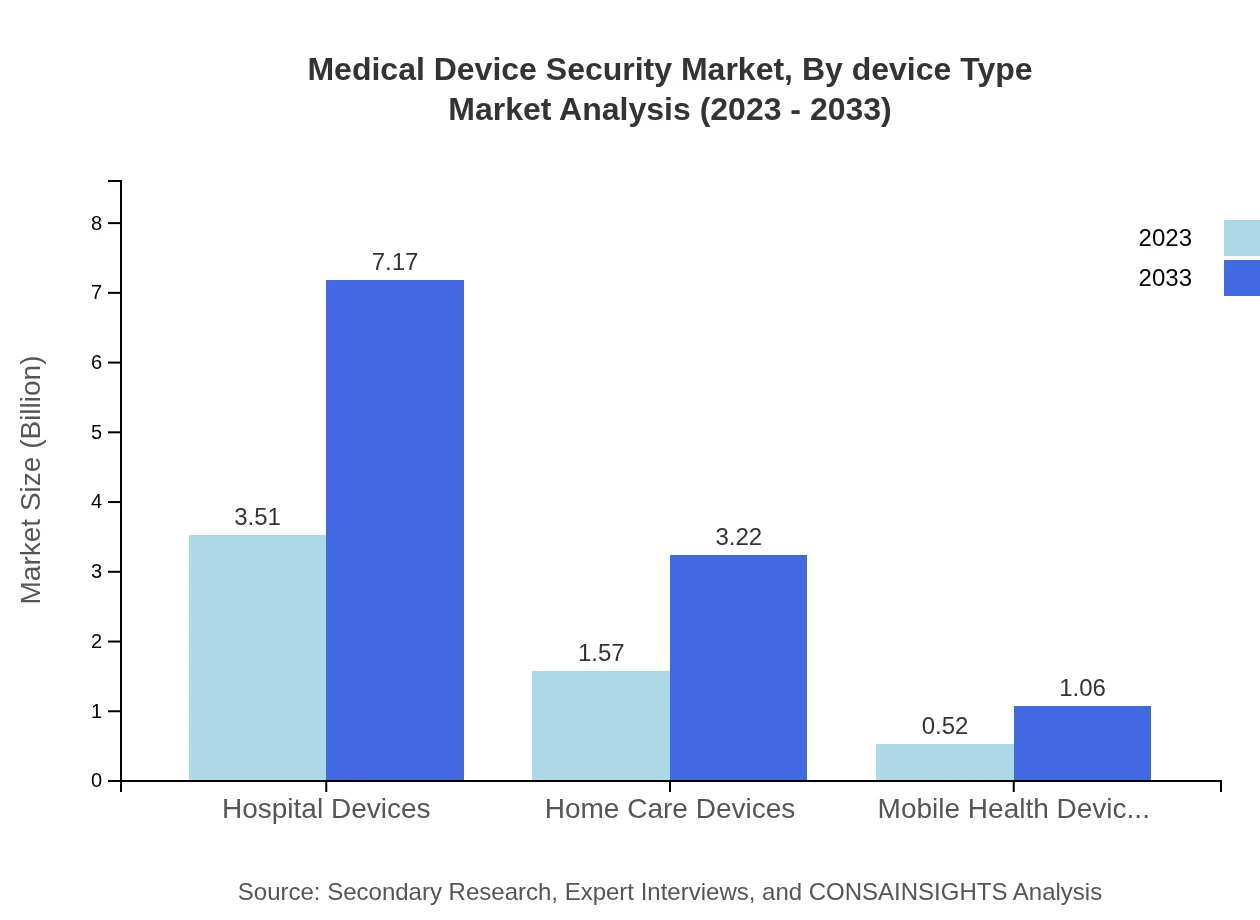
Medical Device Security Market Analysis By Device Type
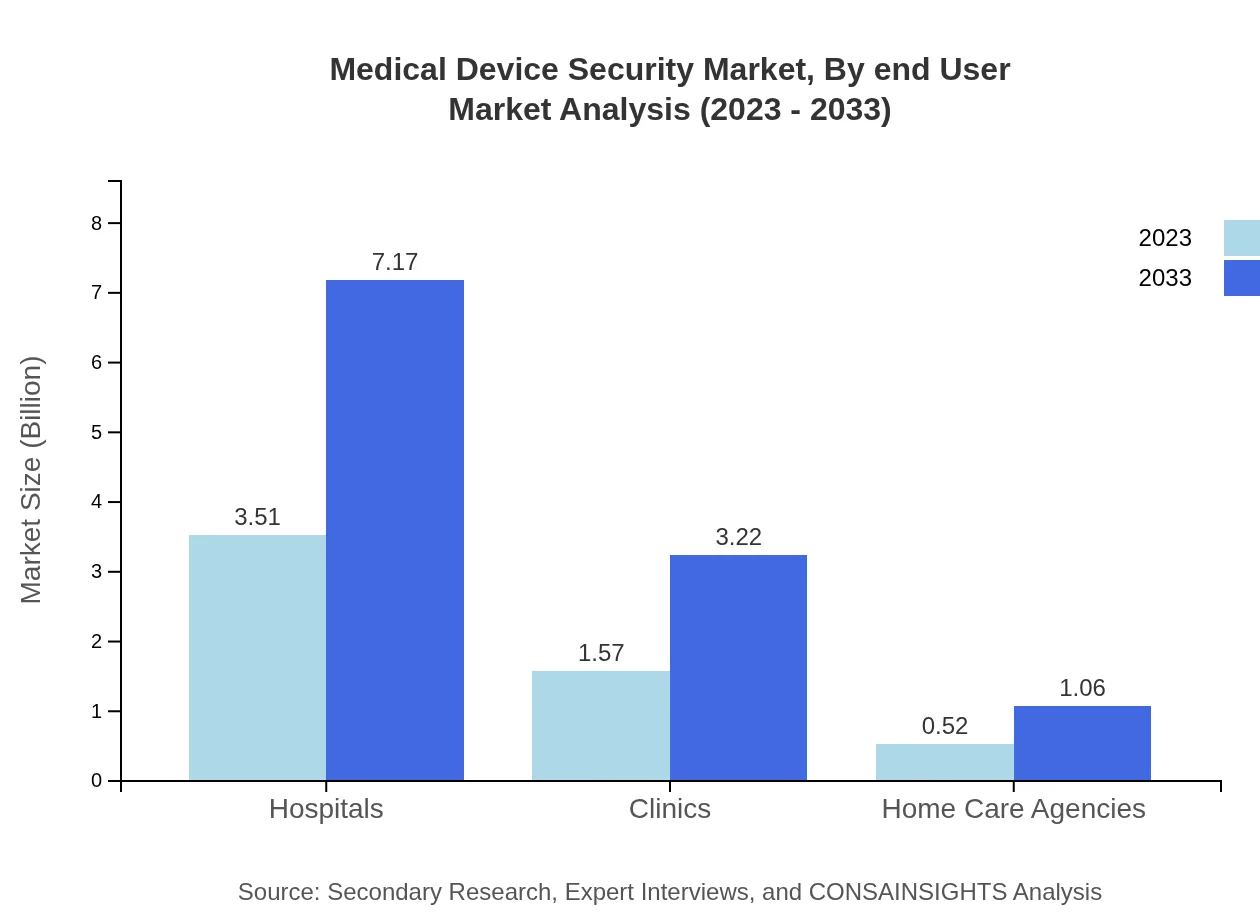
The Medical Device Security market segments by device type consist primarily of hospital devices, which dominate the market with a share of 62.67% in 2023, valued at USD 3.51 billion, growing to USD 7.17 billion by 2033. Home care devices and mobile health devices are also significant segments, with market values projected at USD 1.57 billion and USD 0.52 billion in 2023, respectively.
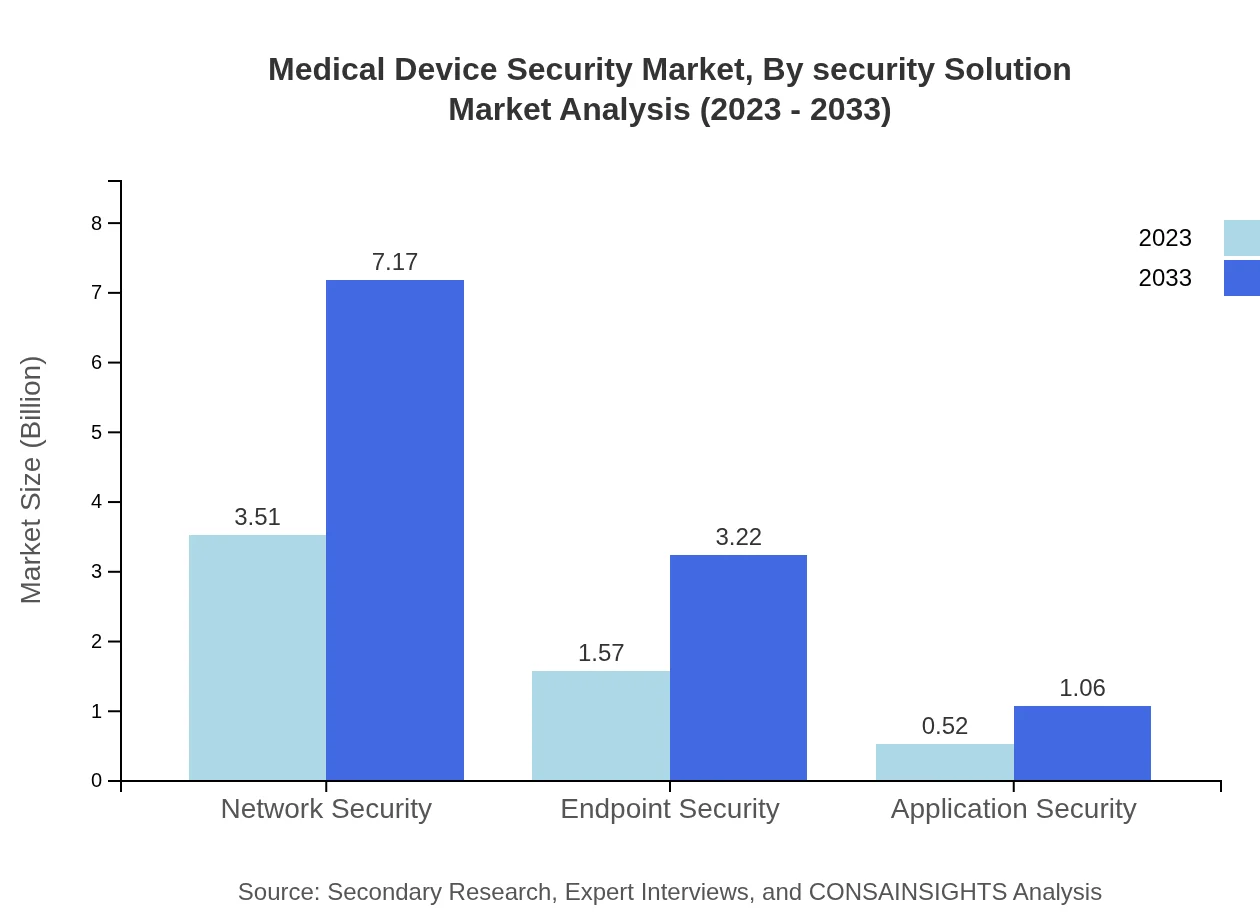
Medical Device Security Market Analysis By Security Solution
In terms of security solutions, network and endpoint security lead the market, collectively accounting for over 90% of the overall revenue. HIPAA compliance requirements drive demand for network security solutions, while endpoint security solutions protect individual devices from cyber threats. Forecasts indicate robust growth in both categories, driven by increasing regulatory pressures and the proliferation of connected devices in healthcare.
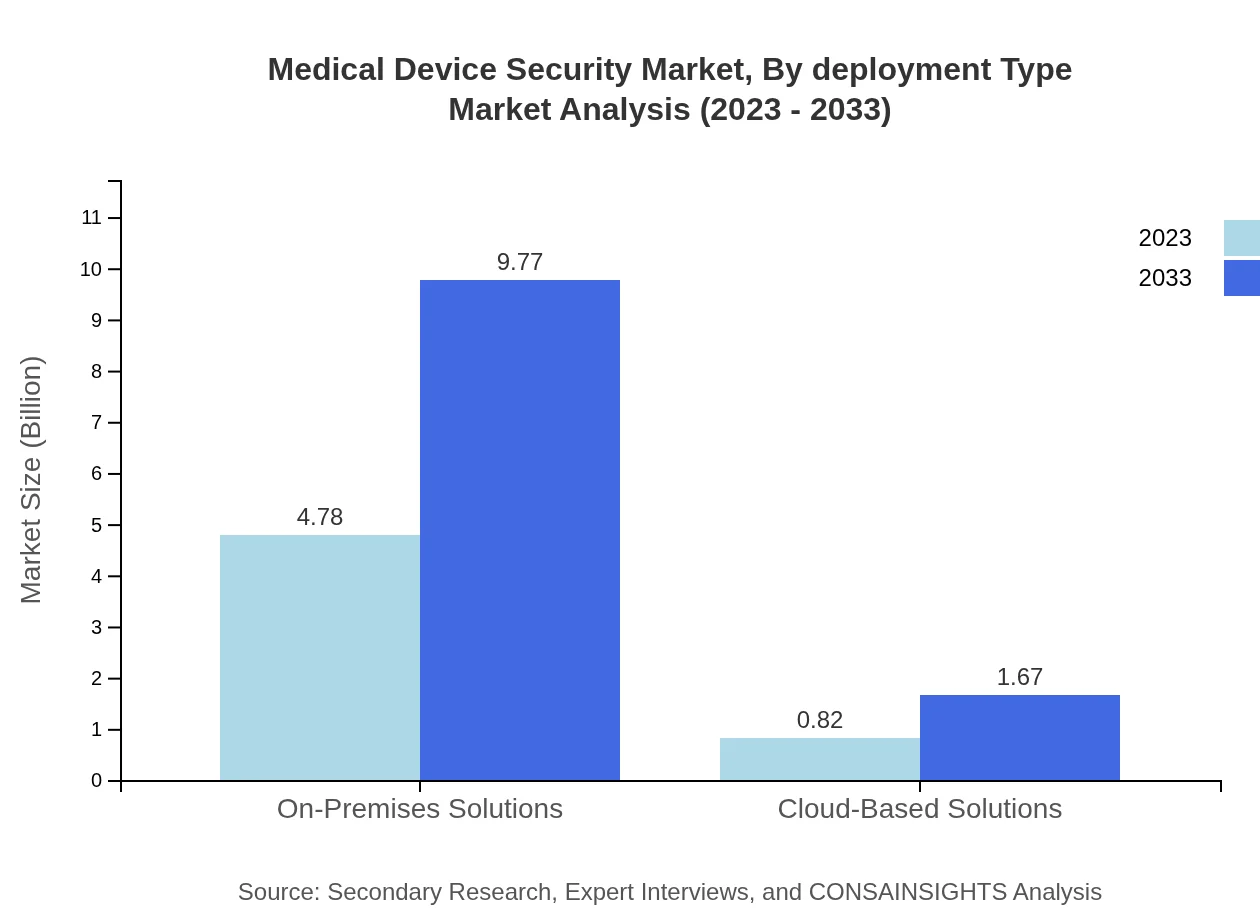
Medical Device Security Market Analysis By Deployment Type
On-premises solutions dominate the Medical Device Security market, holding an 85.37% share in 2023, valued at USD 4.78 billion and projected to reach USD 9.77 billion by 2033. However, cloud-based solutions are gaining traction due to their scalability and cost-effectiveness, expected to grow from USD 0.82 billion to USD 1.67 billion over the same period.
Medical Device Security Market Analysis By End User
Hospitals represent the largest end-user segment, illustrating significant market dominance with a share of 62.67%. The rapid adoption of advanced medical devices and solutions strengthens the demand for security mechanisms in hospitals, while clinics and home care agencies are also important contributors to market growth.
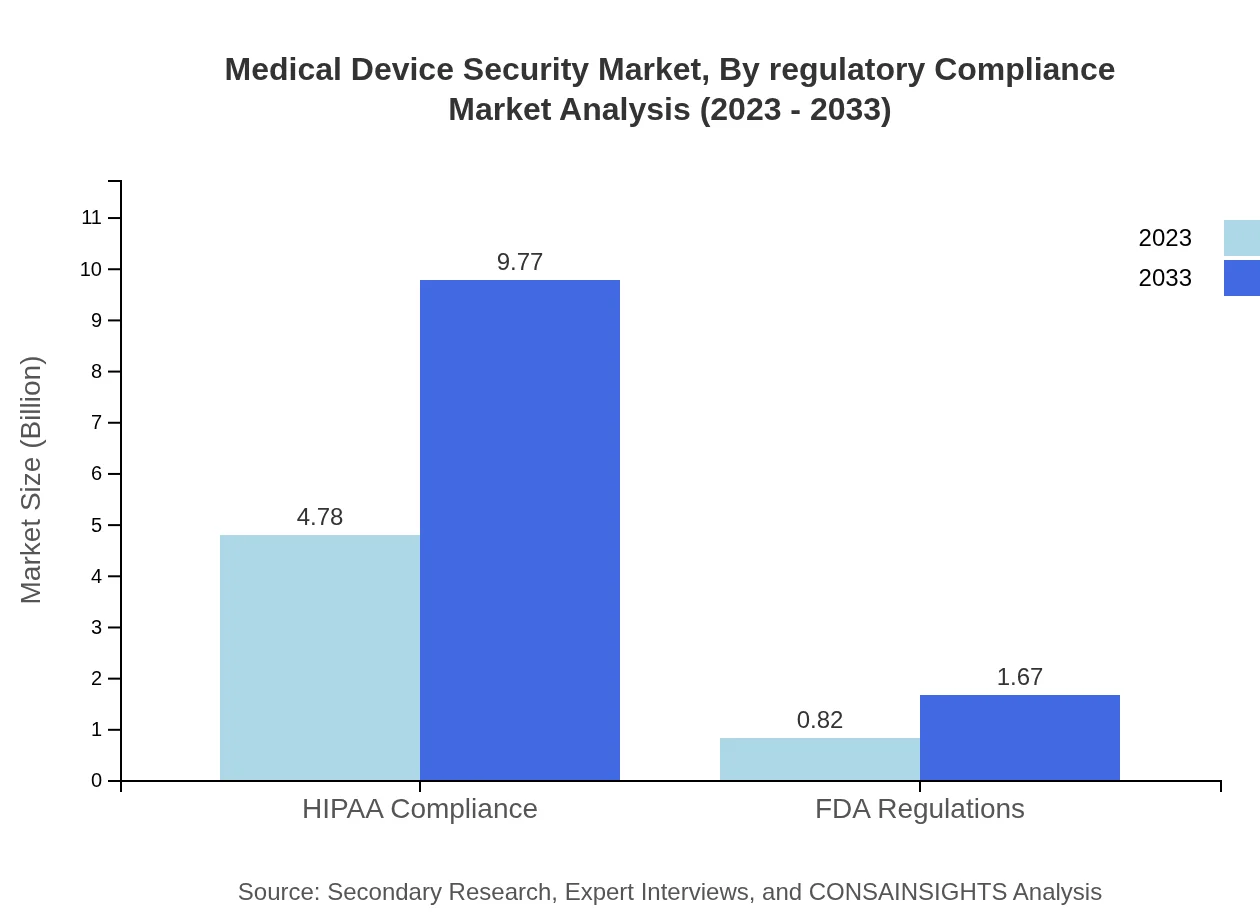
Medical Device Security Market Analysis By Regulatory Compliance
The focus on regulatory compliance, particularly HIPAA regulations, is pivotal in driving the Medical Device Security market. HIPAA compliance solutions are expected to grow from USD 4.78 billion in 2023 to USD 9.77 billion by 2033. Compliance with FDA regulations also holds relevance, reflecting the industry's increasing demand for security against cyber threats.
Medical Device Security Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Medical Device Security Industry
IBM Corporation:
A leading technology company offering cybersecurity solutions tailored for the healthcare sector. Their extensive portfolio includes data protection and threat management systems integrable with medical devices.Medtronic :
A global leader in medical technology, Medtronic is actively enhancing its device security protocols to ensure compliance and protect patient data across their product range.Palo Alto Networks:
Specializes in advanced cybersecurity solutions, including threat prevention particularly targeted at healthcare providers critical in safeguarding medical device networks.Cisco Systems, Inc.:
Provides network security solutions and expertise in managing cyber threats, focusing on ensuring the safety of connected medical devices.Fortinet:
Offers comprehensive cybersecurity solutions, with specific services aimed at protecting healthcare infrastructures and medical devices from emerging cyber threats.We're grateful to work with incredible clients.









FAQs
What is the market size of medical Device Security?
The medical device security market is currently valued at approximately 5.6 billion. With a projected CAGR of 7.2%, it is expected to experience significant growth over the next decade.
What are the key market players or companies in this medical Device Security industry?
Key players in the medical device security market include prominent firms such as McAfee, IBM Security, Palo Alto Networks, and Cisco, who are leading the surge towards enhanced cybersecurity measures.
What are the primary factors driving the growth in the medical Device Security industry?
The growth in the medical device security industry is driven by increasing cyber threats, regulations for healthcare data protection, and the rising adoption of connected medical devices in hospitals and home healthcare.
Which region is the fastest Growing in the medical Device Security?
The fastest-growing region in the medical device security market is Europe, projected to grow from 1.79 billion in 2023 to 3.66 billion by 2033, reflecting a strong demand for security solutions.
Does ConsaInsights provide customized market report data for the medical Device Security industry?
Yes, ConsaInsights offers customized market report data tailored to the medical device security industry, enabling clients to receive specific insights that cater to their business needs.
What deliverables can I expect from this medical Device Security market research project?
Deliverables from the medical device security market research project typically include comprehensive reports, detailed market analysis, competitor assessments, and forecasts based on current trends.
What are the market trends of medical Device Security?
Market trends in medical device security indicate a shift towards advanced technologies such as cloud-based solutions and enhanced network security, reflecting the industry's adaptation to evolving cyber threats.