Clinical Trials Market Report
Published Date: 31 January 2026 | Report Code: clinical-trials
Clinical Trials Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Clinical Trials market from 2023 to 2033, including market size, trends, regional insights, industry analysis, and forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
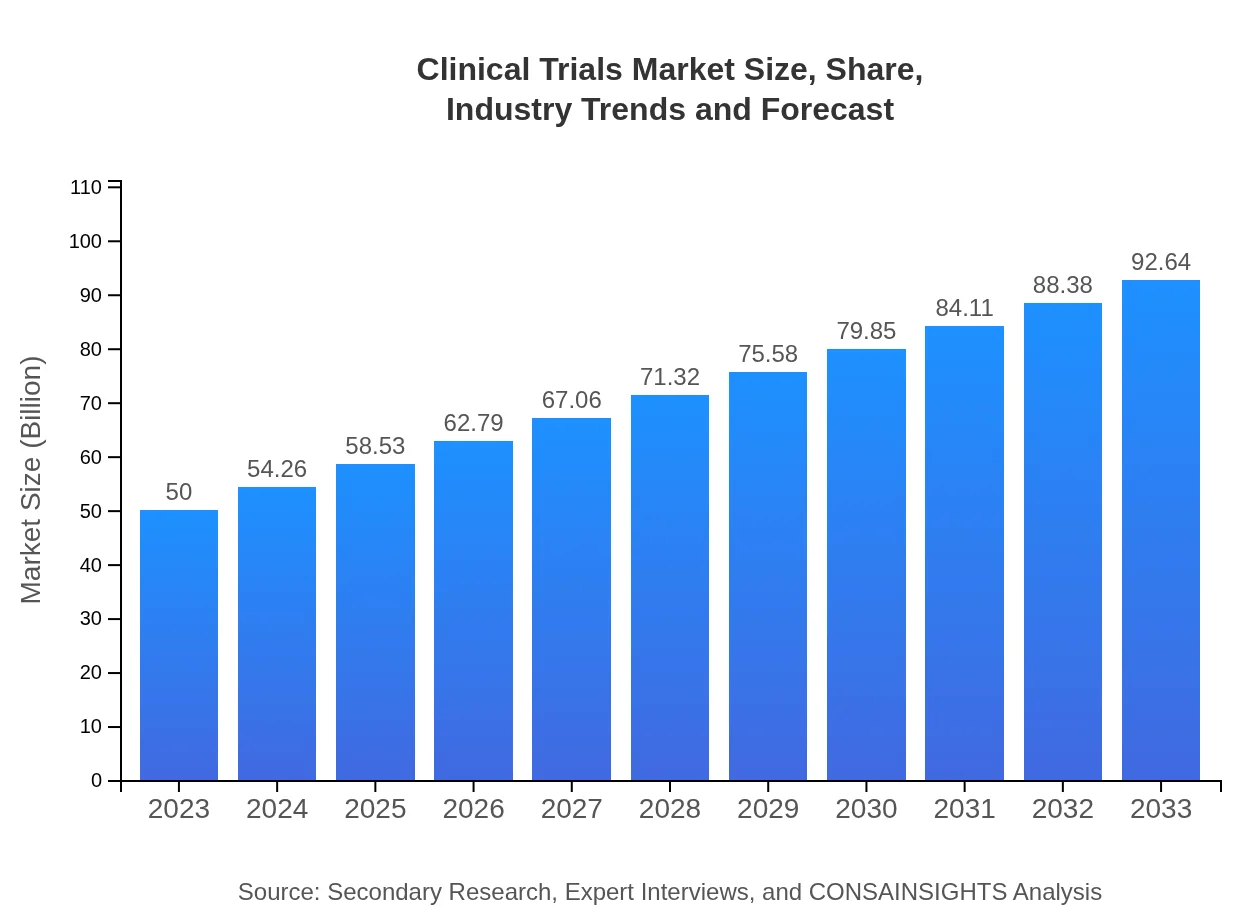
| 2023 Market Size | $50.00 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $92.64 Billion |
| Top Companies | Covance, IQVIA , Parexel, PRA Health Sciences |
| Last Modified Date | 31 January 2026 |
Clinical Trials Market Overview
Customize Clinical Trials Market Report market research report
- ✔ Get in-depth analysis of Clinical Trials market size, growth, and forecasts.
- ✔ Understand Clinical Trials's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Clinical Trials
What is the Market Size & CAGR of Clinical Trials market in 2023?
Clinical Trials Industry Analysis
Clinical Trials Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Clinical Trials Market Analysis Report by Region
Europe Clinical Trials Market Report:
The European Clinical Trials market is forecasted to grow from $14.77 billion in 2023 to $27.37 billion by 2033. The region benefits from a well-established healthcare infrastructure and collaborative partnerships between stakeholders to accelerate drug development.Asia Pacific Clinical Trials Market Report:
The Asia Pacific region is witnessing significant growth in the Clinical Trials market, projected to increase from $8.79 billion in 2023 to $16.28 billion by 2033. Factors contributing to this growth include a large patient base, increasing healthcare investments, and favorable regulatory policies promoting clinical research.North America Clinical Trials Market Report:
North America, leading the Clinical Trials market with a value of $19.23 billion in 2023, is projected to reach $35.62 billion by 2033. This growth is attributed to a robust pharmaceutical industry, advanced research facilities, and high R&D expenditures.South America Clinical Trials Market Report:
The South American Clinical Trials market is expected to grow from $3.65 billion in 2023 to $6.77 billion by 2033. The growth is driven by an emerging market for drug discovery and an increase in clinical research activities supported by local governments.Middle East & Africa Clinical Trials Market Report:
The Clinical Trials market in the Middle East and Africa is anticipated to increase from $3.56 billion in 2023 to $6.61 billion by 2033, propelled by a rise in clinical research initiatives and a growing number of clinical trial registries in the region.Tell us your focus area and get a customized research report.
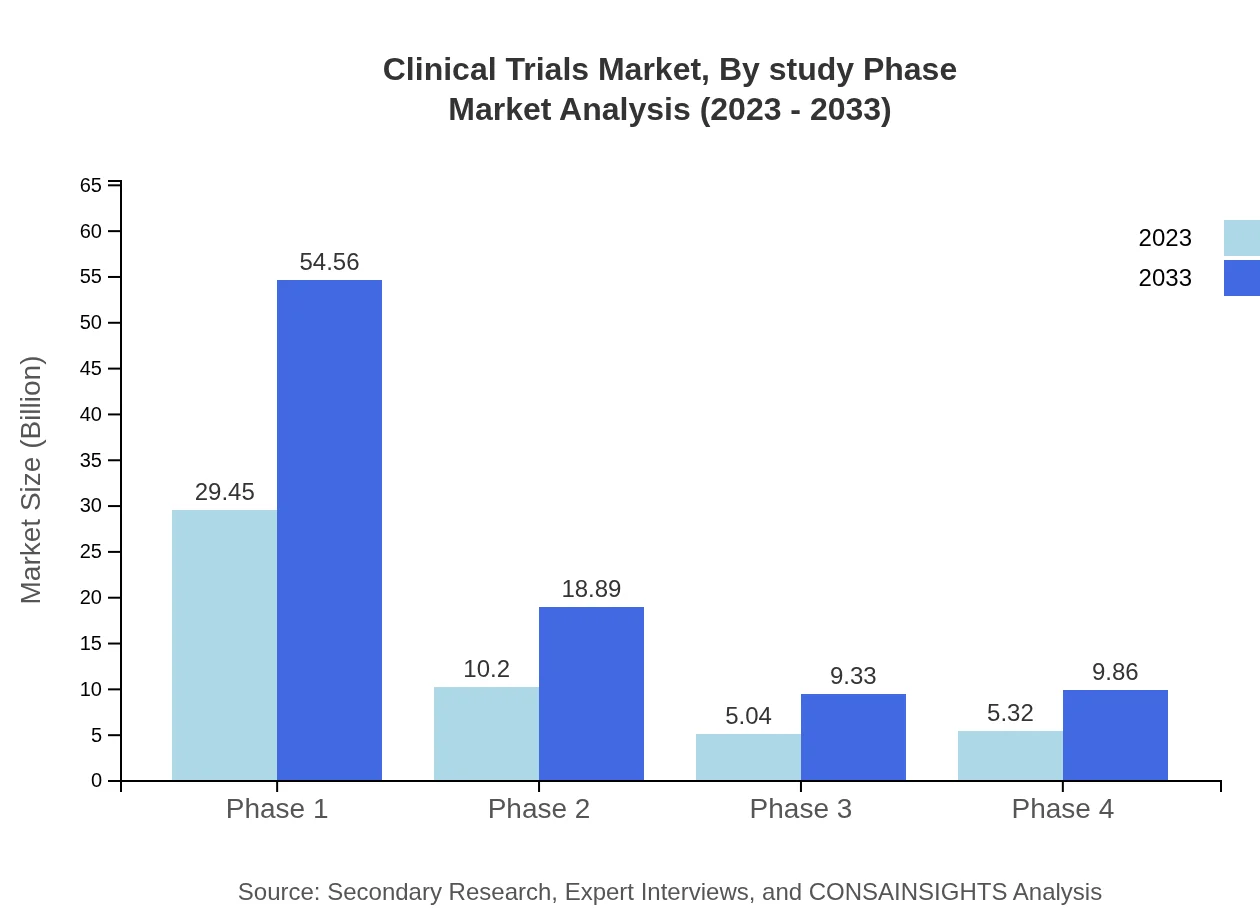
Clinical Trials Market Analysis By Study Phase
In 2023, Phase 1 trials dominate the Clinical Trials market at $29.45 billion, expected to grow to $54.56 billion by 2033. Phase 2 trials follow at $10.20 billion and are estimated to reach $18.89 billion, while Phase 3 and Phase 4 trials are projected to grow from $5.04 billion and $5.32 billion respectively, to $9.33 billion and $9.86 billion by 2033.
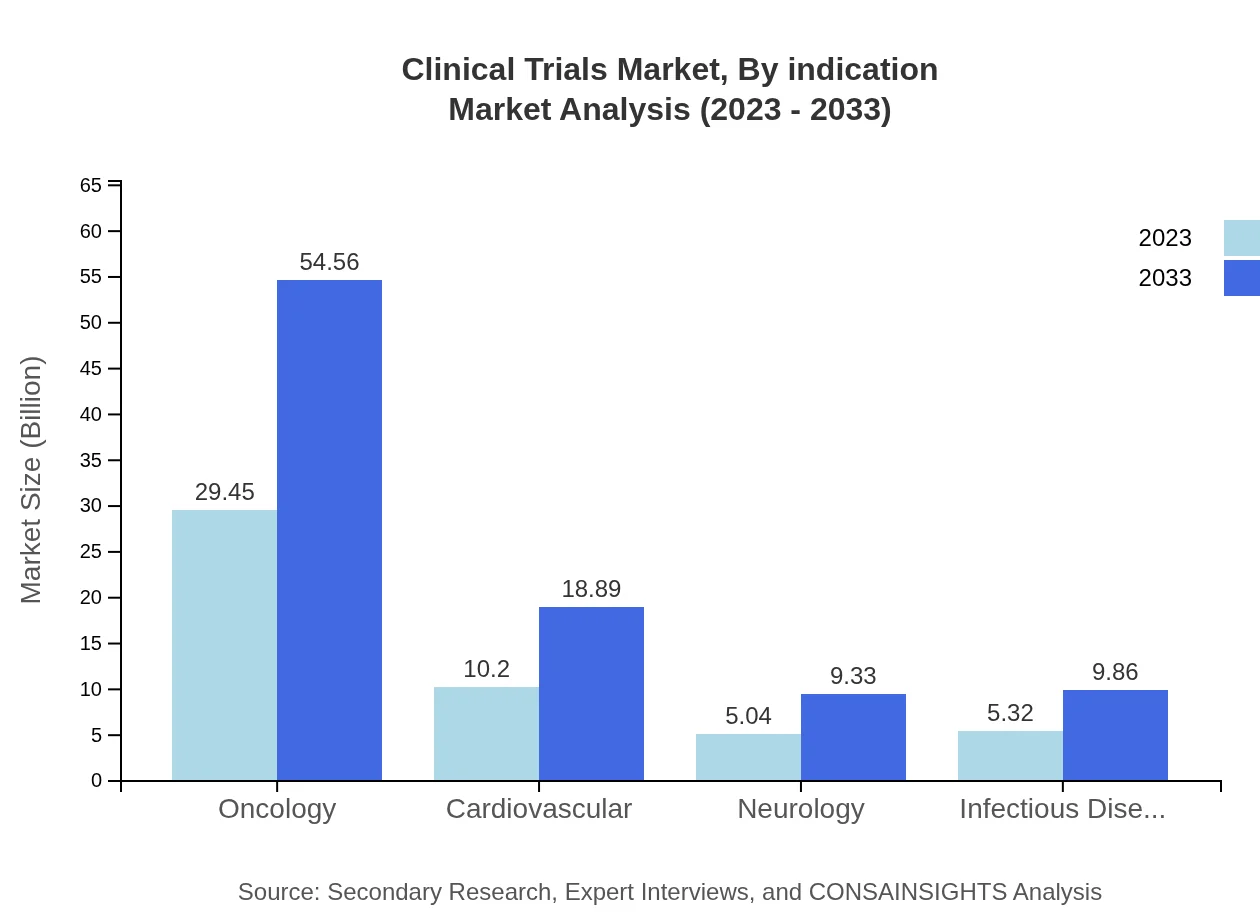
Clinical Trials Market Analysis By Indication
Oncology remains the largest segment in the Clinical Trials market, standing at $29.45 billion in 2023, projected to rise to $54.56 billion by 2033. Cardiovascular trials hold a value of $10.20 billion, expected to expand to $18.89 billion. Neurology and Infectious Diseases also contribute to the market with their respective projections of $5.04 billion to $9.33 billion, and $5.32 billion to $9.86 billion.
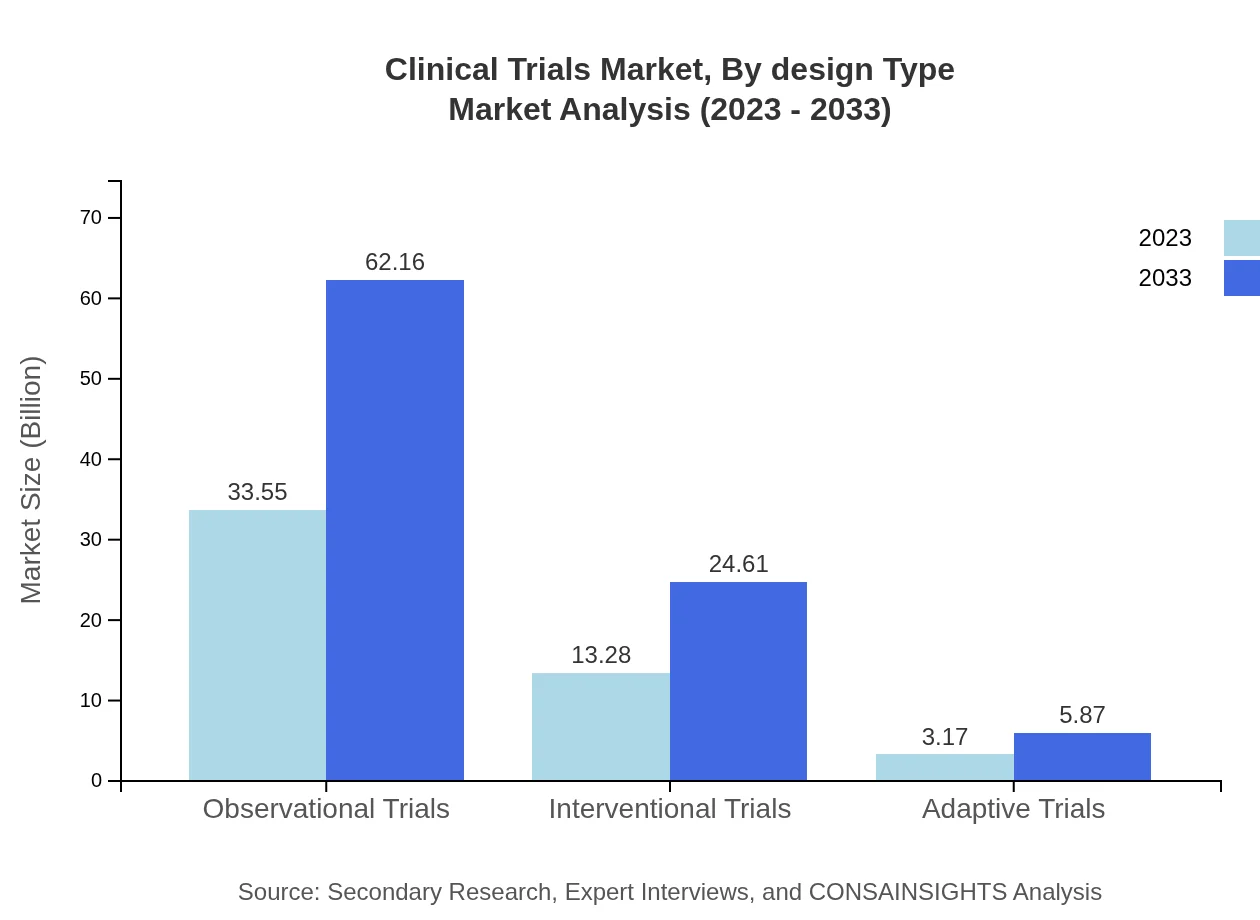
Clinical Trials Market Analysis By Design Type
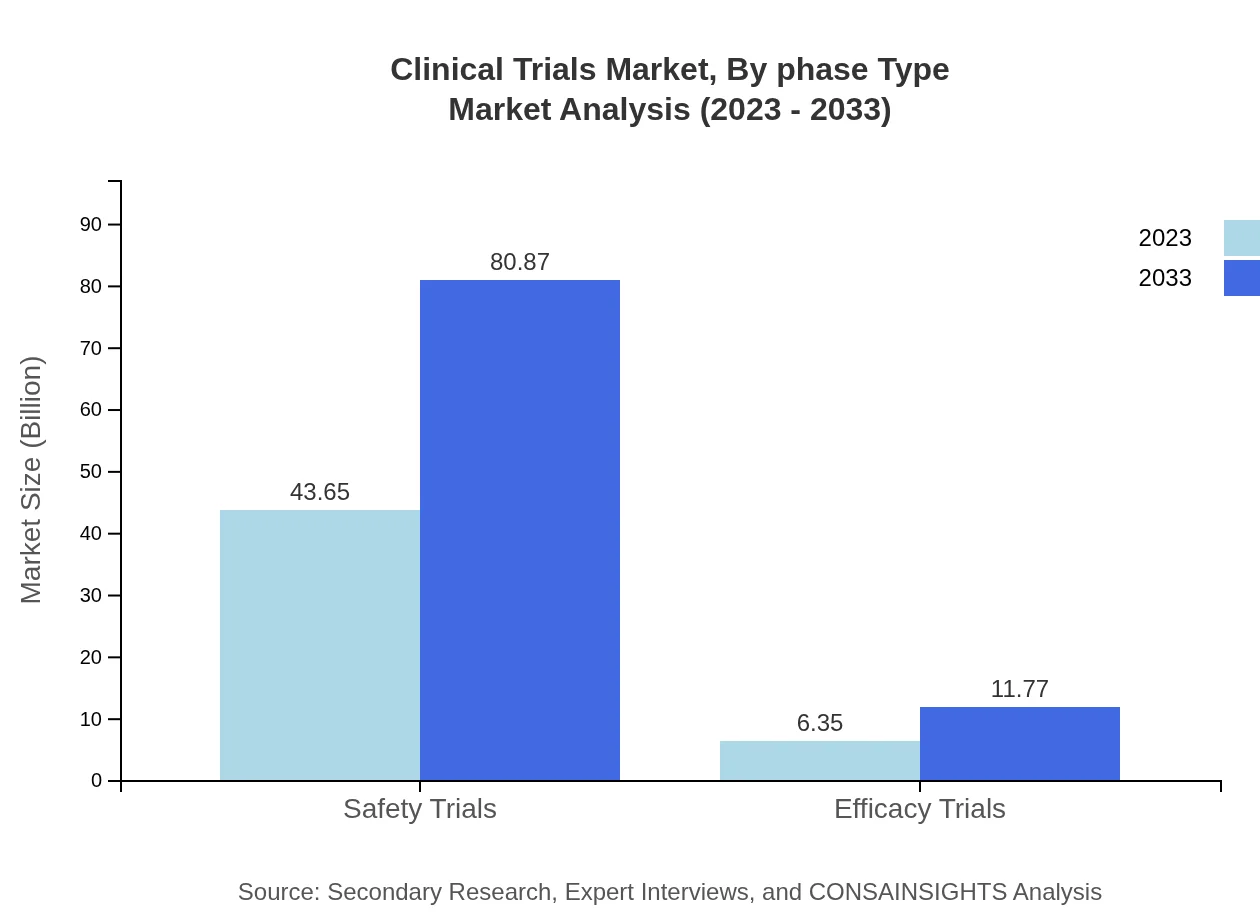
Safety trials command a large market share of $43.65 billion in 2023, with a steep rise to $80.87 billion anticipated by 2033. Other significant segments include observational trials, worth $33.55 billion in 2023, which is expected to grow to $62.16 billion, and interventional trials, helping to diversify the research landscape.
Clinical Trials Market Analysis By Phase Type
The market segmentation by phase types showcases Phase 1 trials leading at a value of $29.45 billion in 2023, doubling to $54.56 billion by 2033. Phase 2 trials account for $10.20 billion with projections of $18.89 billion, alongside Phase 3 and Phase 4 trials that contribute significantly to the total market values.
Clinical Trials Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Clinical Trials Industry
Covance:
Covance is a global leader in drug development and commercialization services, providing comprehensive laboratory and clinical trial solutions to biopharmaceutical clients.IQVIA :
IQVIA leverages advanced data analytics and technological solutions to support pharmaceutical development, uniquely positioning itself in the Clinical Trials landscape.Parexel:
Parexel is a multinational CRO providing specialized services in drug development and regulatory consulting, known for its deep therapeutic expertise across various indications.PRA Health Sciences:
PRA Health Sciences specializes in providing outsourced clinical development solutions, ensuring efficient and timely trial execution while maintaining compliance with regulatory demands.We're grateful to work with incredible clients.









FAQs
What is the market size of clinical trials?
The global clinical trials market is valued at approximately $50 billion in 2023, with a projected compound annual growth rate (CAGR) of 6.2%, forecasted to reach significant growth through 2033.
What are the key market players or companies in this clinical trials industry?
Key players in the clinical trials industry include major pharmaceutical companies, contract research organizations (CROs), and biotechnology firms that drive innovation and oversee various trial phases.
What are the primary factors driving the growth in the clinical trials industry?
Key growth drivers include increasing demand for new therapies, expanding patient populations, advances in technology, and regulatory support for faster trial approvals, enhancing the pipeline of effective treatments.
Which region is the fastest Growing in the clinical trials?
North America is the fastest-growing region in the clinical trials market, projected to expand from $19.23 billion in 2023 to $35.62 billion by 2033, indicating strong market potential and investment.
Does ConsaInsights provide customized market report data for the clinical trials industry?
Yes, ConsaInsights offers customized market reports tailored to client specifications, providing in-depth analyses that address unique strategic goals within the clinical trials sector.
What deliverables can I expect from this clinical trials market research project?
Deliverables include a comprehensive market analysis report, insights on growth trends, competitive landscape, segment breakdowns, and forecasts, ensuring informed decision-making and strategic planning.
What are the market trends of clinical trials?
Current trends in the clinical trials market include increased focus on patient-centric approaches, adoption of decentralized trial methodologies, and utilization of data analytics to enhance trial outcomes.