Fetal Heart Rate Monitoring Device Market Report
Published Date: 31 January 2026 | Report Code: fetal-heart-rate-monitoring-device
Fetal Heart Rate Monitoring Device Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the fetal heart rate monitoring device market, detailing key insights, trends, and growth forecasts from 2023 to 2033. It presents in-depth market segmentation, regional performance, and technological advancements reshaping the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
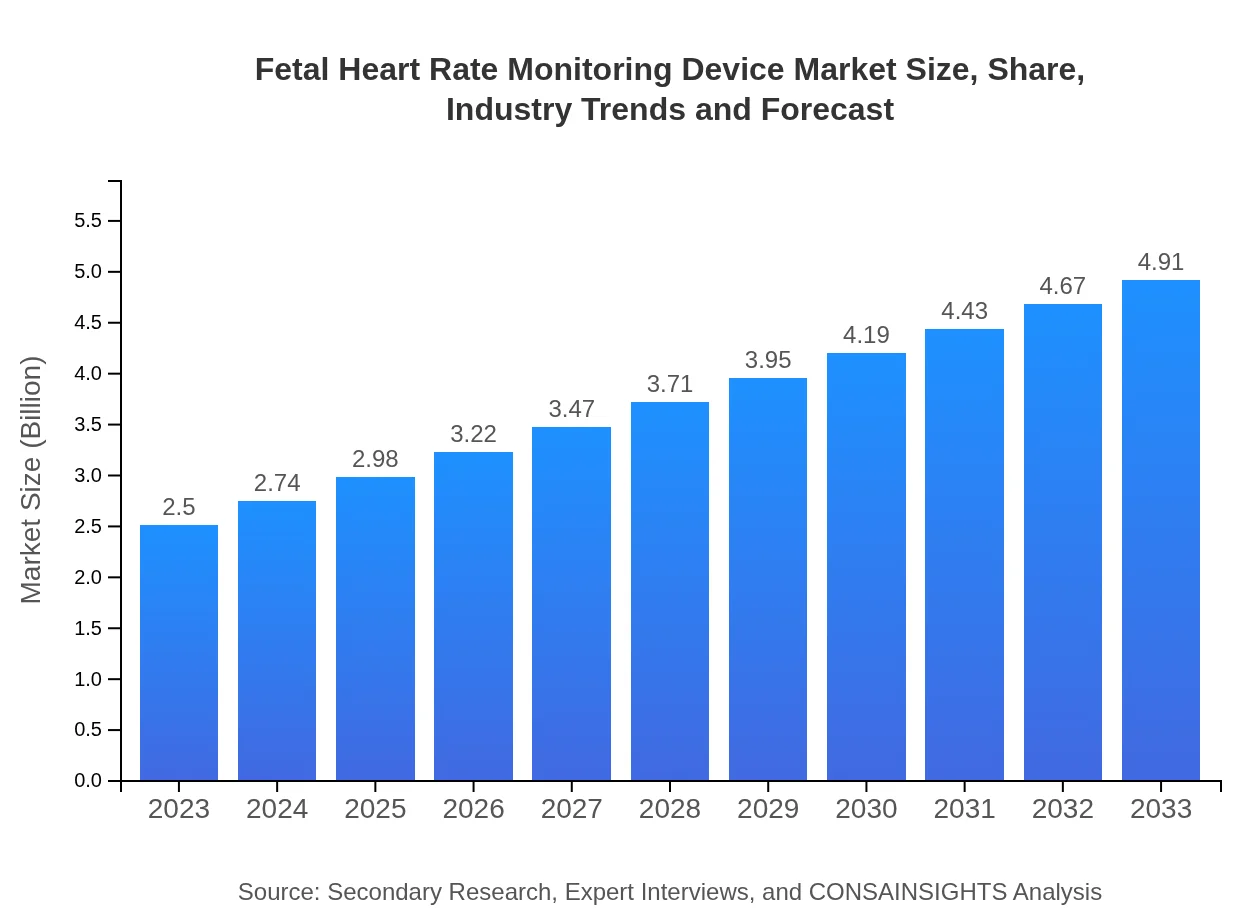
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | GE Healthcare, Philips Healthcare, Medtronic , Natus Medical, Cardinal Health |
| Last Modified Date | 31 January 2026 |
Fetal Heart Rate Monitoring Device Market Overview
Customize Fetal Heart Rate Monitoring Device Market Report market research report
- ✔ Get in-depth analysis of Fetal Heart Rate Monitoring Device market size, growth, and forecasts.
- ✔ Understand Fetal Heart Rate Monitoring Device's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Fetal Heart Rate Monitoring Device
What is the Market Size & CAGR of Fetal Heart Rate Monitoring Device market in 2023?
Fetal Heart Rate Monitoring Device Industry Analysis
Fetal Heart Rate Monitoring Device Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Fetal Heart Rate Monitoring Device Market Analysis Report by Region
Europe Fetal Heart Rate Monitoring Device Market Report:
The European fetal heart rate monitoring device market is projected to grow from $0.75 billion in 2023 to $1.47 billion by 2033. Growth factors include stringent healthcare regulations ensuring high-quality patient care and an increase in births across the region. Countries like Germany, France, and the UK are significant contributors to regional market growth.Asia Pacific Fetal Heart Rate Monitoring Device Market Report:
The Asia Pacific market for fetal heart rate monitoring devices is projected to grow significantly from $0.53 billion in 2023 to $1.03 billion in 2033, fueled by improving healthcare infrastructure, increasing awareness of prenatal care, and rising disposable incomes. Countries like India and China are witnessing rapid adoption of advanced monitoring technologies, driving growth in the region.North America Fetal Heart Rate Monitoring Device Market Report:
North America is expected to dominate the market, scaling from $0.83 billion in 2023 to $1.64 billion in 2033. High prevalence of complications during pregnancy, coupled with advanced healthcare facilities and presence of established companies, attributes to this growth. The United States is a primary driver of innovation and adoption of monitoring technologies in this region.South America Fetal Heart Rate Monitoring Device Market Report:
In South America, the fetal heart monitoring device market is expected to increase from $0.20 billion in 2023 to $0.40 billion by 2033. Factors contributing to this growth include increased investment in healthcare systems and growing awareness of maternal-fetal health. Brazil and Argentina are likely to emerge as key markets within this region.Middle East & Africa Fetal Heart Rate Monitoring Device Market Report:
The Middle East and Africa market is anticipated to grow modestly, from $0.19 billion in 2023 to $0.37 billion in 2033. Improvements in healthcare infrastructure and patient management systems in countries such as South Africa and UAE are significant growth drivers. However, challenges related to healthcare accessibility may pose risks to rapid market expansion.Tell us your focus area and get a customized research report.
Fetal Heart Rate Monitoring Device Market Analysis By Product
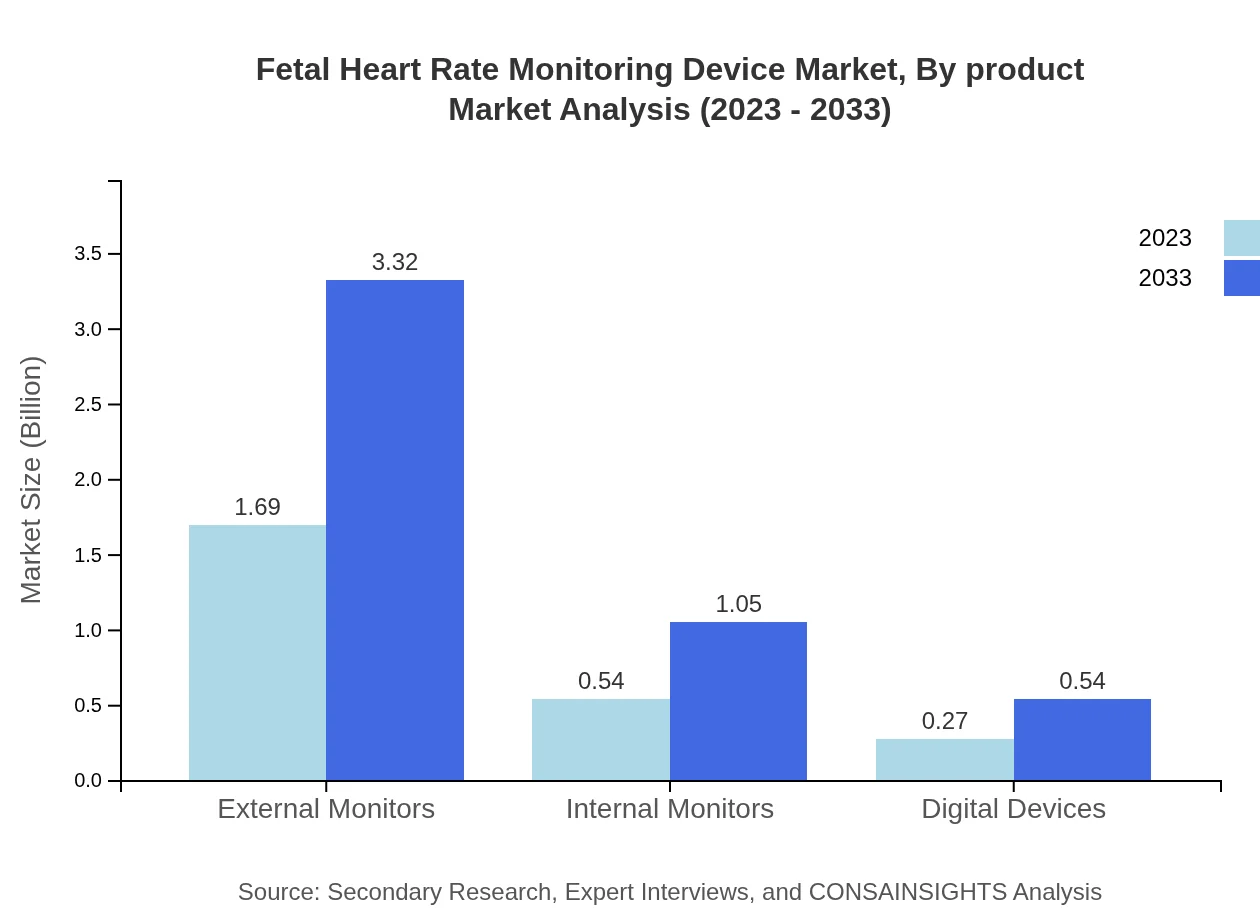
The fetal heart rate monitoring device market by product is segmented into external monitors, internal monitors, and digital devices. External monitors accounted for a market size of $1.69 billion in 2023 and are projected to double to $3.32 billion by 2033, holding a significant market share due to their non-invasiveness and ease of use. Internal monitors are also showing growth, with projections moving from $0.54 billion to $1.05 billion, alongside digital devices growing from $0.27 billion to $0.54 billion in the same interval.
Fetal Heart Rate Monitoring Device Market Analysis By Application
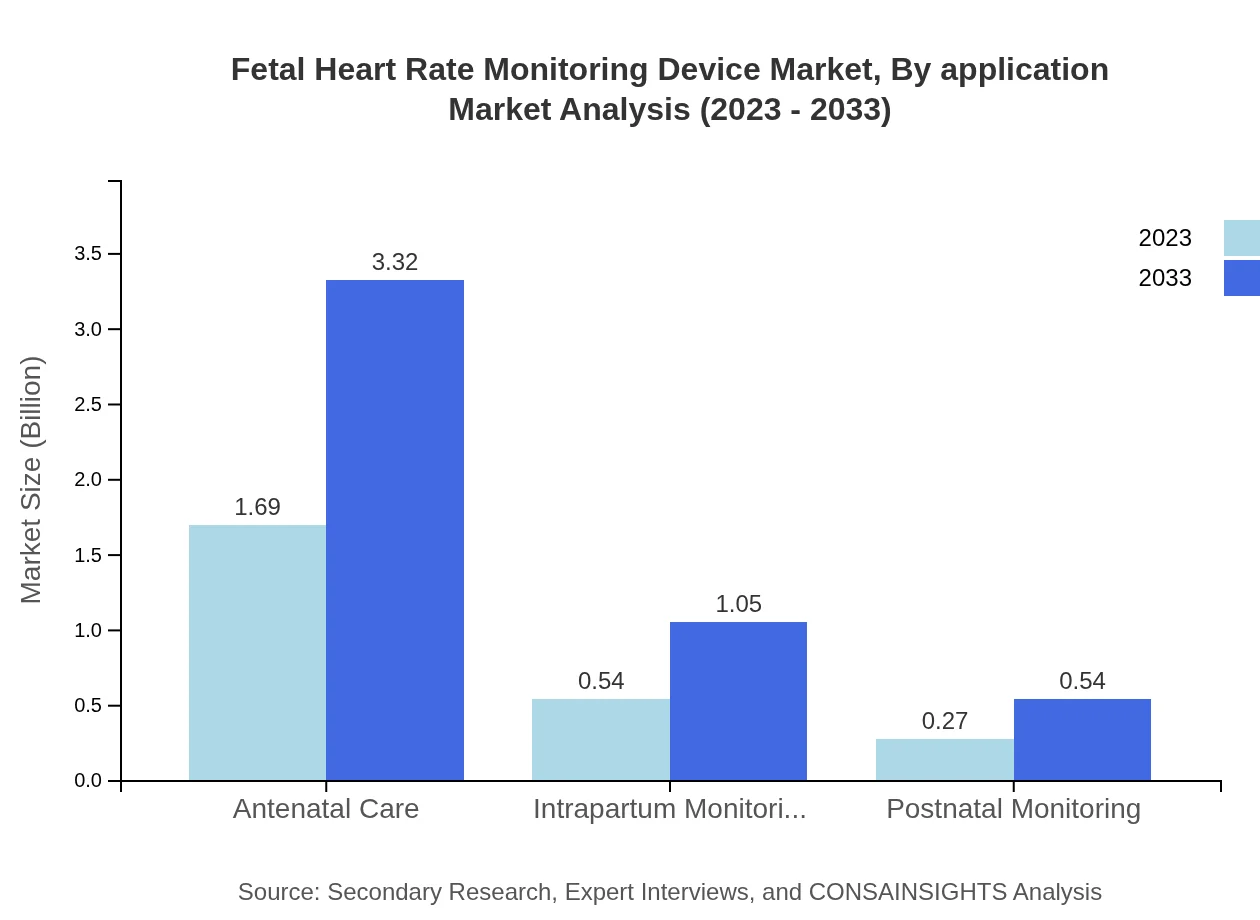
By application, the market can be divided into antenatal, intrapartum, and postnatal monitoring. Antenatal monitoring will dominate with size moving from $1.69 billion in 2023 to $3.32 billion by 2033. Intrapartum monitoring and postnatal care application segments will also report significant growth, increasing from $0.54 billion to $1.05 billion and $0.27 billion to $0.54 billion respectively.
Fetal Heart Rate Monitoring Device Market Analysis By End User
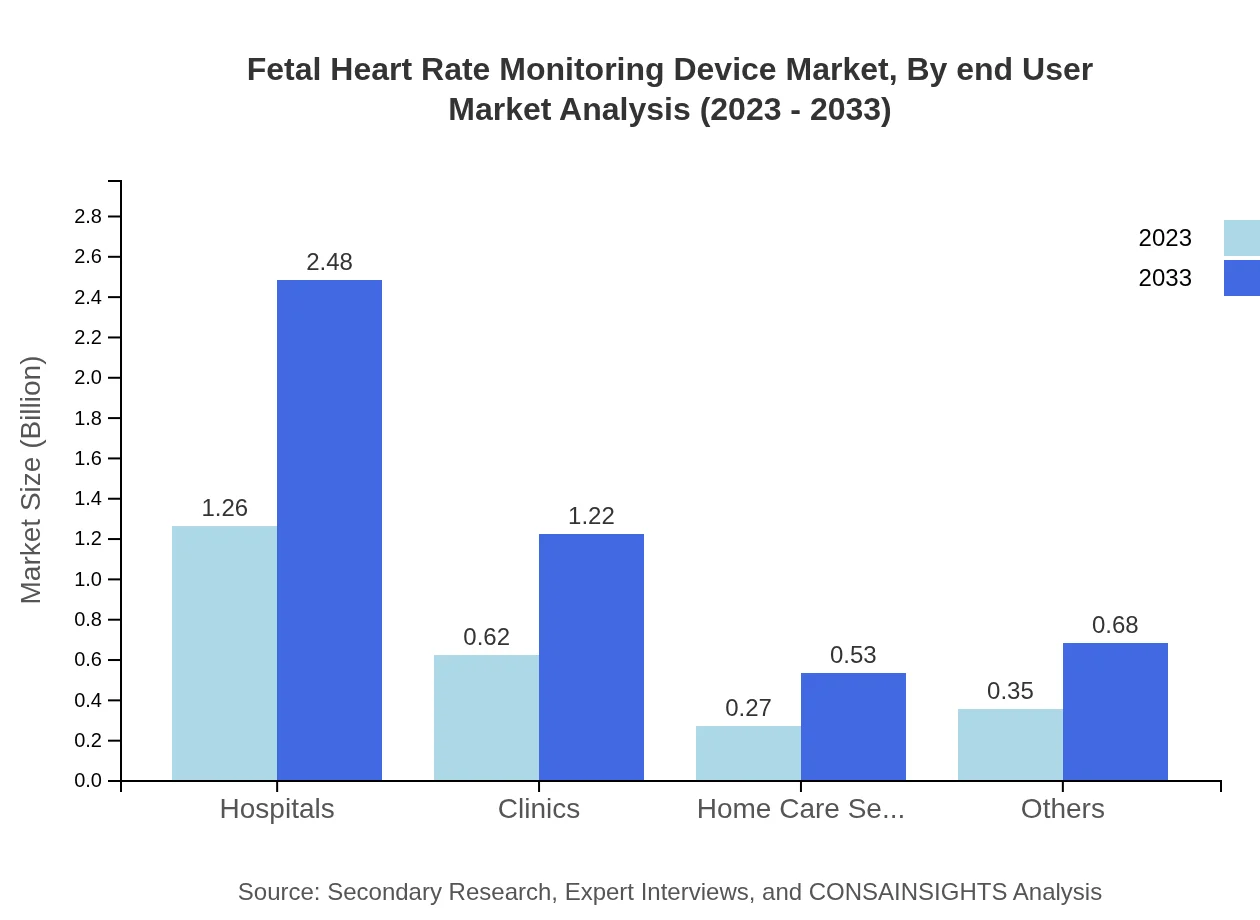
The end-user segmentation reveals that hospitals are the largest consumers, growing from $1.26 billion in 2023 to $2.48 billion by 2033, reflecting the growing demands of obstetric departments. Clinics and home care settings represent substantial markets as well, with further growth driven by increasing shifts towards outpatient care and telehealth solutions.
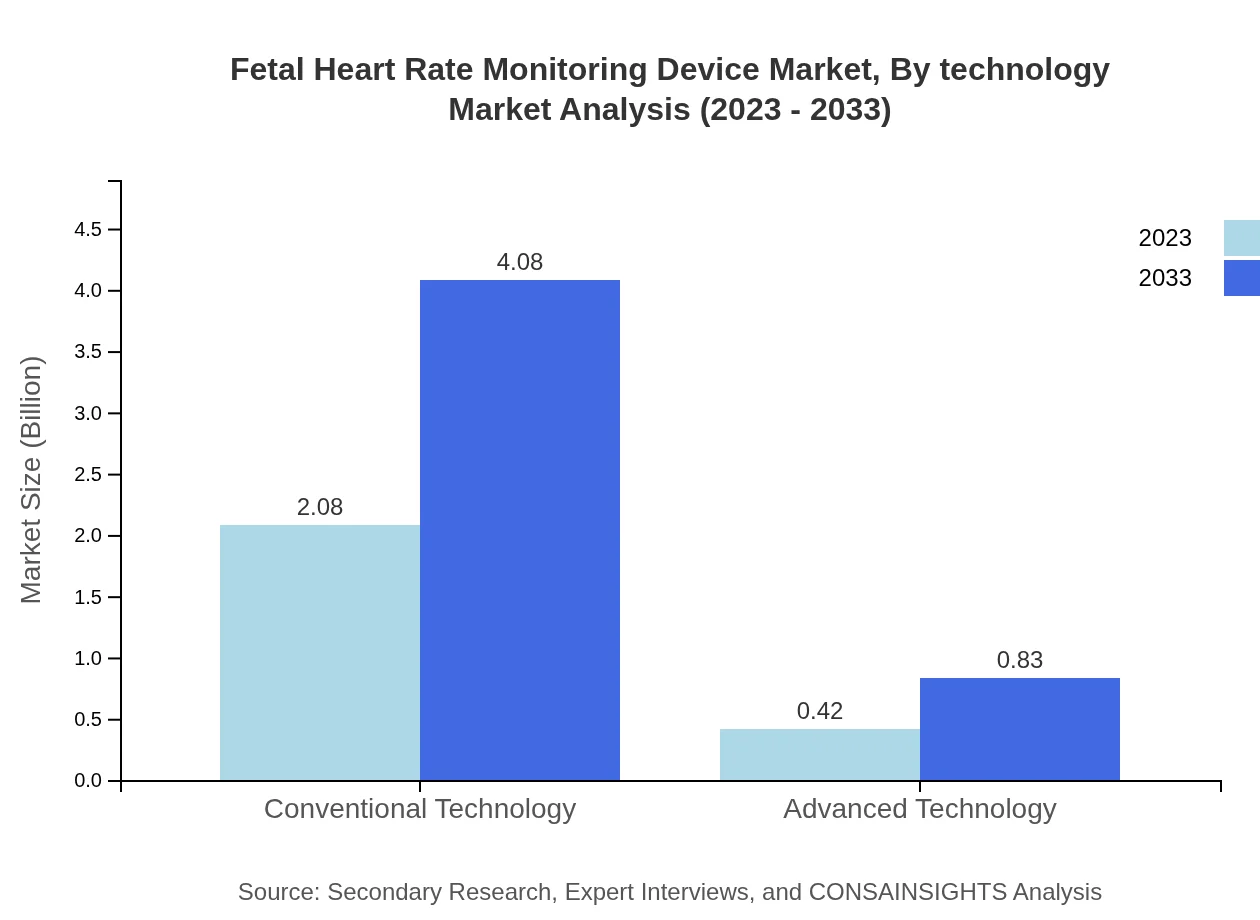
Fetal Heart Rate Monitoring Device Market Analysis By Technology
The technology segment is primarily categorized into conventional and advanced technologies. Conventional technology currently leads the market, valued at $2.08 billion and likely to grow to $4.08 billion by 2033. Conversely, advanced technology is also gaining traction, expected to climb from $0.42 billion to $0.83 billion as hospitals and clinics adopt more efficient methods for monitoring.
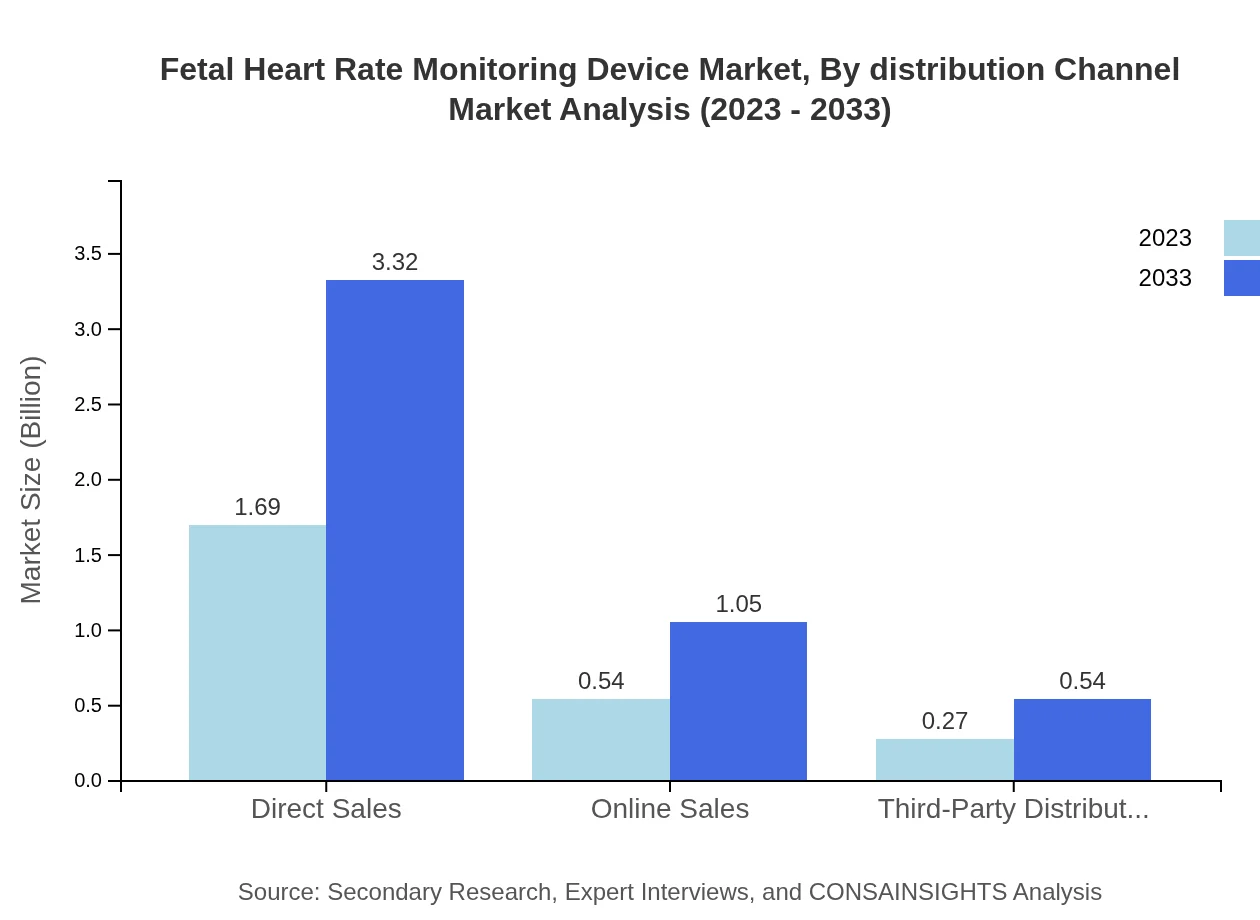
Fetal Heart Rate Monitoring Device Market Analysis By Distribution Channel
The market distribution channels segment includes direct sales, online sales, and third-party distributors. Direct sales are prominent, increasing from $1.69 billion in 2023 to $3.32 billion by 2033. Online sales will reflect robust growth as consumers shift toward e-commerce, rising from $0.54 billion to $1.05 billion, and third-party distributors will witness modest increment from $0.27 billion to $0.54 billion.
Fetal Heart Rate Monitoring Device Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Fetal Heart Rate Monitoring Device Industry
GE Healthcare:
GE Healthcare is a leading player in the medical technology sector, offering innovative fetal heart rate monitoring solutions with a focus on quality and patient care.Philips Healthcare:
Philips Healthcare specializes in imaging systems and monitoring technologies, providing advanced solutions to improve the overall healthcare experience for mothers and infants.Medtronic :
Medtronic offers a range of medical products, including fetal monitoring devices, emphasizing improving the health of patients and enhancing clinical outcomes.Natus Medical:
Natus Medical is a company that focuses on neonatal, sleep, and hearing solutions in the healthcare market, recognized for their contributions to fetal monitoring.Cardinal Health:
Cardinal Health is involved in the distribution of medical products, including fetal monitoring devices, and plays a vital role in ensuring patient access to quality care.We're grateful to work with incredible clients.









FAQs
What is the market size of fetal Heart Rate Monitoring Device?
The fetal heart rate monitoring device market was valued at approximately 2.5 billion in 2023 and is expected to grow at a CAGR of 6.8% from 2023 to 2033. This growth will elevate the market size significantly by 2033.
What are the key market players or companies in this fetal Heart Rate Monitoring Device industry?
Key players in the fetal heart rate monitoring device industry include GE Healthcare, Philips, Siemens Healthineers, and Drägerwerk. These companies are leading the market with innovative technologies and product offerings aimed at enhancing maternal and fetal care.
What are the primary factors driving the growth in the fetal Heart Rate Monitoring Device industry?
The market growth is driven by technological advancements in monitoring devices, increasing awareness of prenatal care, the rising incidence of high-risk pregnancies, and a growing emphasis on maternal and fetal health globally.
Which region is the fastest Growing in the fetal Heart Rate Monitoring device?
The fastest-growing region in the fetal heart rate monitoring device market is North America, projected to grow from 0.83 billion in 2023 to 1.64 billion by 2033, driven by advanced healthcare infrastructure and increasing demand for fetal monitoring.
Does ConsInsights provide customized market report data for the fetal Heart Rate Monitoring Device industry?
Yes, ConsInsights offers customized market report data tailored to specific needs within the fetal heart rate monitoring device industry. This service allows clients to obtain precise insights based on unique business requirements.
What deliverables can I expect from this fetal Heart Rate Monitoring Device market research project?
From this market research project, you can expect detailed market analysis reports, growth forecasts, competitive landscape assessments, segment-wise data, and regional insights, thus providing a comprehensive understanding of the fetal heart rate monitoring device market.
What are the market trends of fetal Heart Rate Monitoring Device?
Trends in the fetal heart rate monitoring device market include a shift towards digital monitoring devices, enhanced use of telehealth for continuous monitoring, and increased incorporation of portable and user-friendly technology to improve prenatal care.