Urinary Incontinence Treatment Devices Market Report
Published Date: 31 January 2026 | Report Code: urinary-incontinence-treatment-devices
Urinary Incontinence Treatment Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Urinary Incontinence Treatment Devices market, covering market size, growth forecasts, regional dynamics, and latest trends for the period 2023 - 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
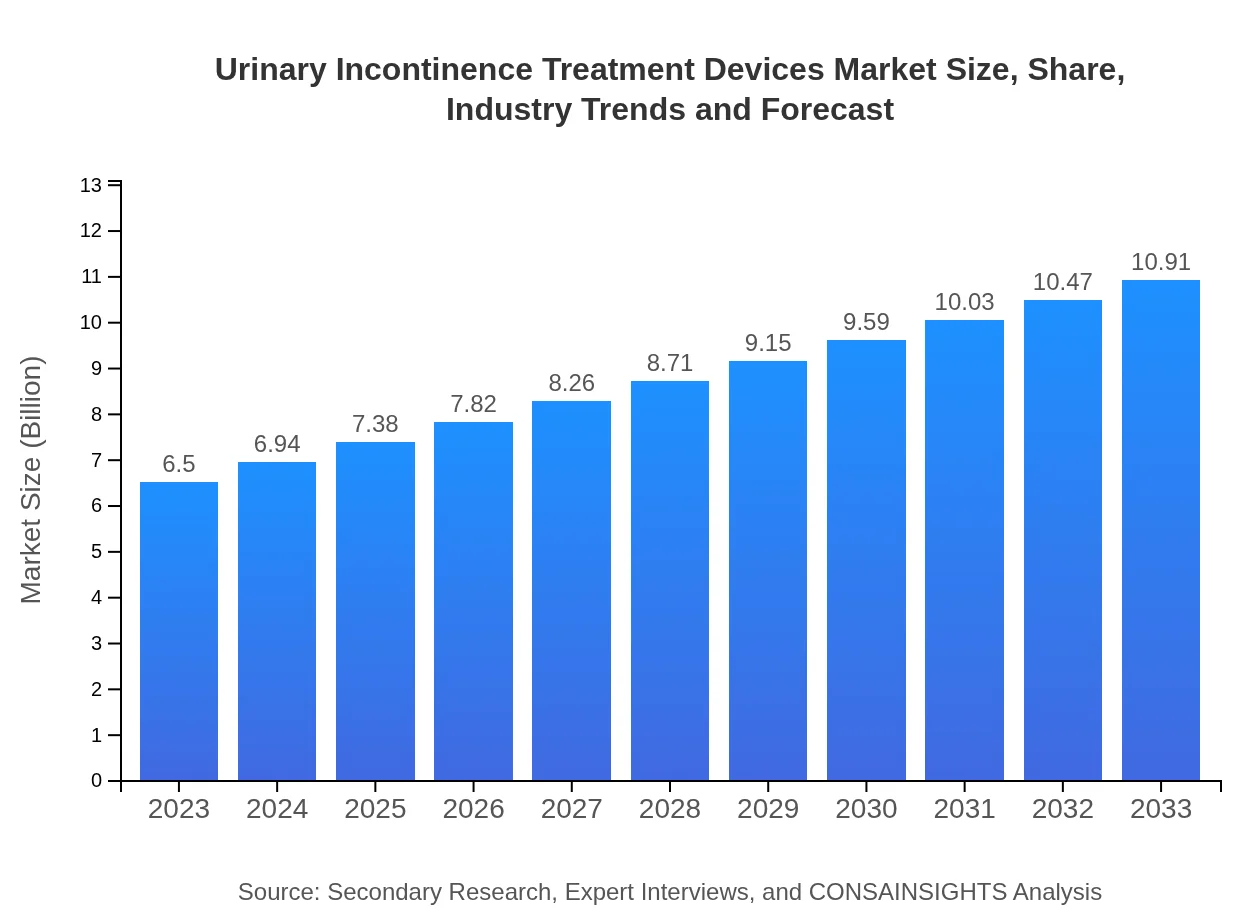
| 2023 Market Size | $6.50 Billion |
| CAGR (2023-2033) | 5.2% |
| 2033 Market Size | $10.91 Billion |
| Top Companies | Medtronic , Coloplast A/S, Boston Scientific Corporation, Astellas Pharma Inc., Stryker Corporation |
| Last Modified Date | 31 January 2026 |
Urinary Incontinence Treatment Devices Market Overview
Customize Urinary Incontinence Treatment Devices Market Report market research report
- ✔ Get in-depth analysis of Urinary Incontinence Treatment Devices market size, growth, and forecasts.
- ✔ Understand Urinary Incontinence Treatment Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Urinary Incontinence Treatment Devices
What is the Market Size & CAGR of Urinary Incontinence Treatment Devices market in 2023?
Urinary Incontinence Treatment Devices Industry Analysis
Urinary Incontinence Treatment Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Urinary Incontinence Treatment Devices Market Analysis Report by Region
Europe Urinary Incontinence Treatment Devices Market Report:
The European market was valued at $1.70 billion in 2023 and is anticipated to grow to $2.85 billion by 2033. Factors such as supportive government initiatives, innovative treatment methodologies, and high patient engagement significantly contribute to this growth.Asia Pacific Urinary Incontinence Treatment Devices Market Report:
In the Asia Pacific region, the market was valued at $1.26 billion in 2023 and is projected to grow to $2.11 billion by 2033. Factors such as increasing healthcare investments, growing population density, and rising awareness about urinary incontinence treatment options are boosting market demand.North America Urinary Incontinence Treatment Devices Market Report:
North America has the largest share in the market, valued at $2.53 billion in 2023, expected to reach $4.24 billion by 2033. The region's high market share is driven by advanced healthcare facilities, high awareness levels, and strong insurance coverage for urinary incontinence treatments.South America Urinary Incontinence Treatment Devices Market Report:
South America's market size reached $0.19 billion in 2023, with a forecast growth to $0.32 billion by 2033. This growth is attributed to enhancing healthcare infrastructure and a surge in awareness campaigns aimed at educating the population about urinary incontinence.Middle East & Africa Urinary Incontinence Treatment Devices Market Report:
In the Middle East and Africa, the market size for urinary incontinence treatment devices was $0.83 billion in 2023, expanding to $1.39 billion by 2033. The growth in this region is influenced by improving healthcare systems and increasing patient awareness regarding urinary incontinence.Tell us your focus area and get a customized research report.
Urinary Incontinence Treatment Devices Market Analysis By Device Type
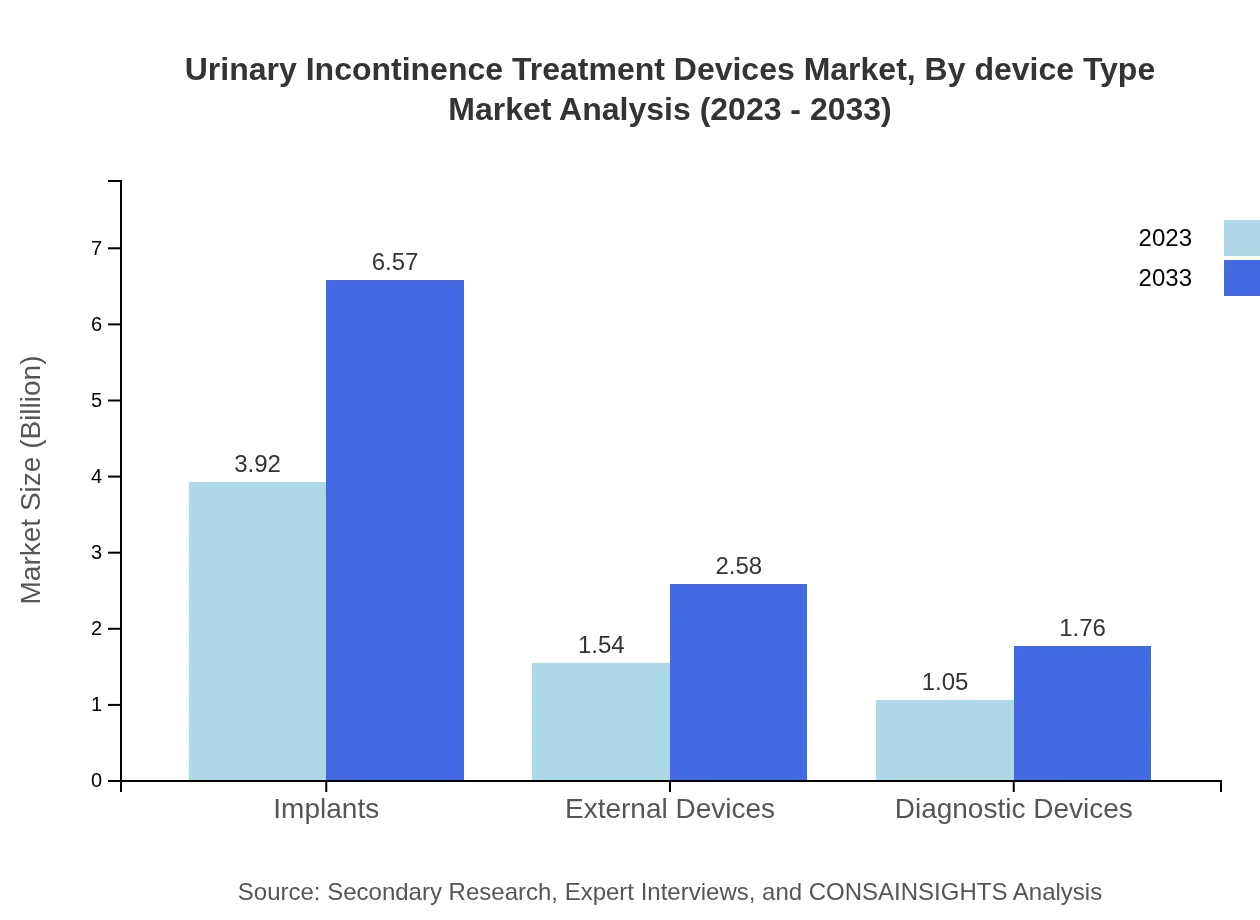
The market segmentation by device type includes implants, external devices, and diagnostic devices. Implants accounted for a significant market share of $3.92 billion in 2023, increasing to $6.57 billion by 2033, while external devices generated $1.54 billion, growing to $2.58 billion. The advanced features and comfort provided by these devices are key drivers of their popularity in the treatment of urinary incontinence.
Urinary Incontinence Treatment Devices Market Analysis By Technology
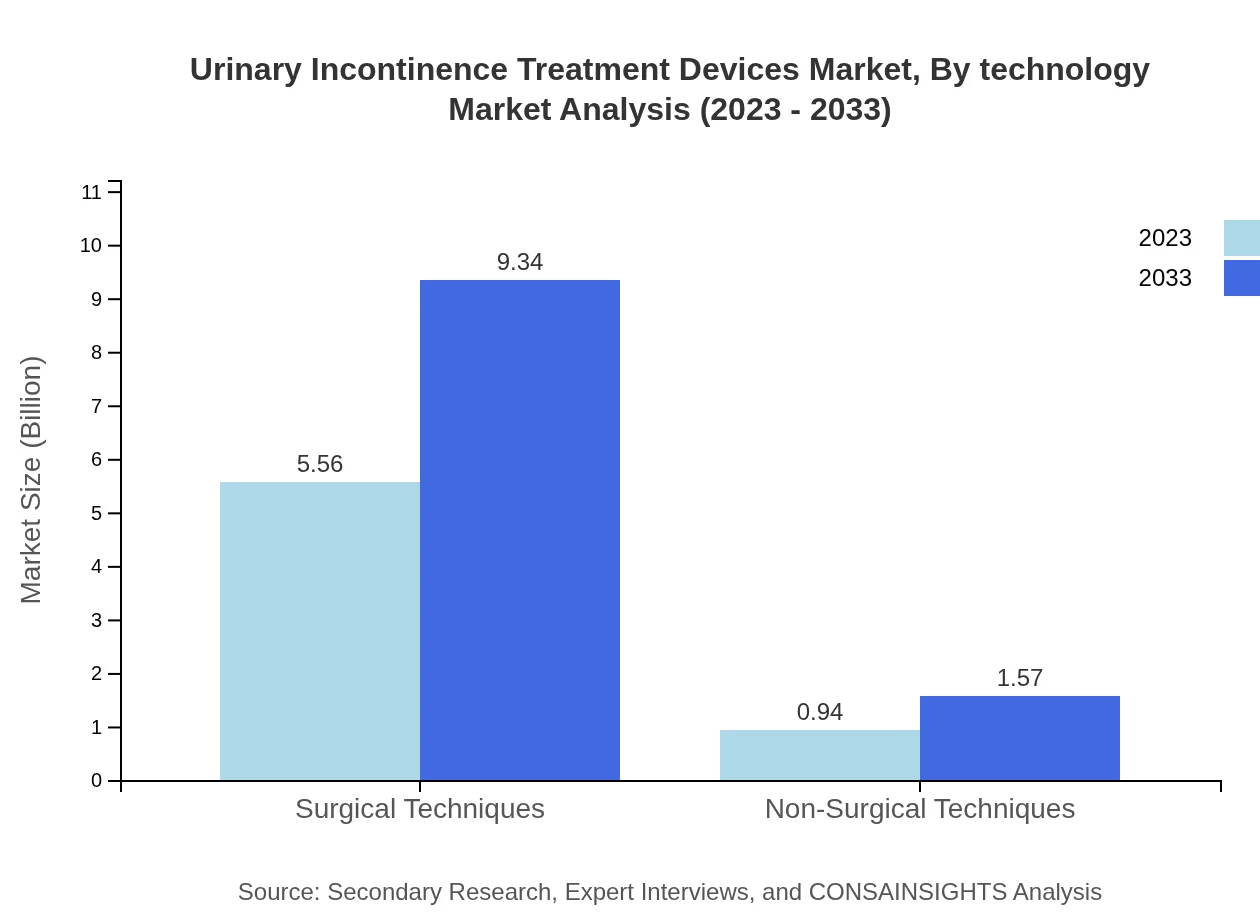
In terms of technology, the urinary incontinence treatment devices market is distinguished between surgical techniques and non-surgical techniques. Surgical techniques commanded a market size of $5.56 billion in 2023, expected to reach $9.34 billion by 2033. Non-surgical techniques, though smaller, illustrate a growing acceptance with a market size of $0.94 billion in 2023 and anticipated growth to $1.57 billion.
Urinary Incontinence Treatment Devices Market Analysis By End User
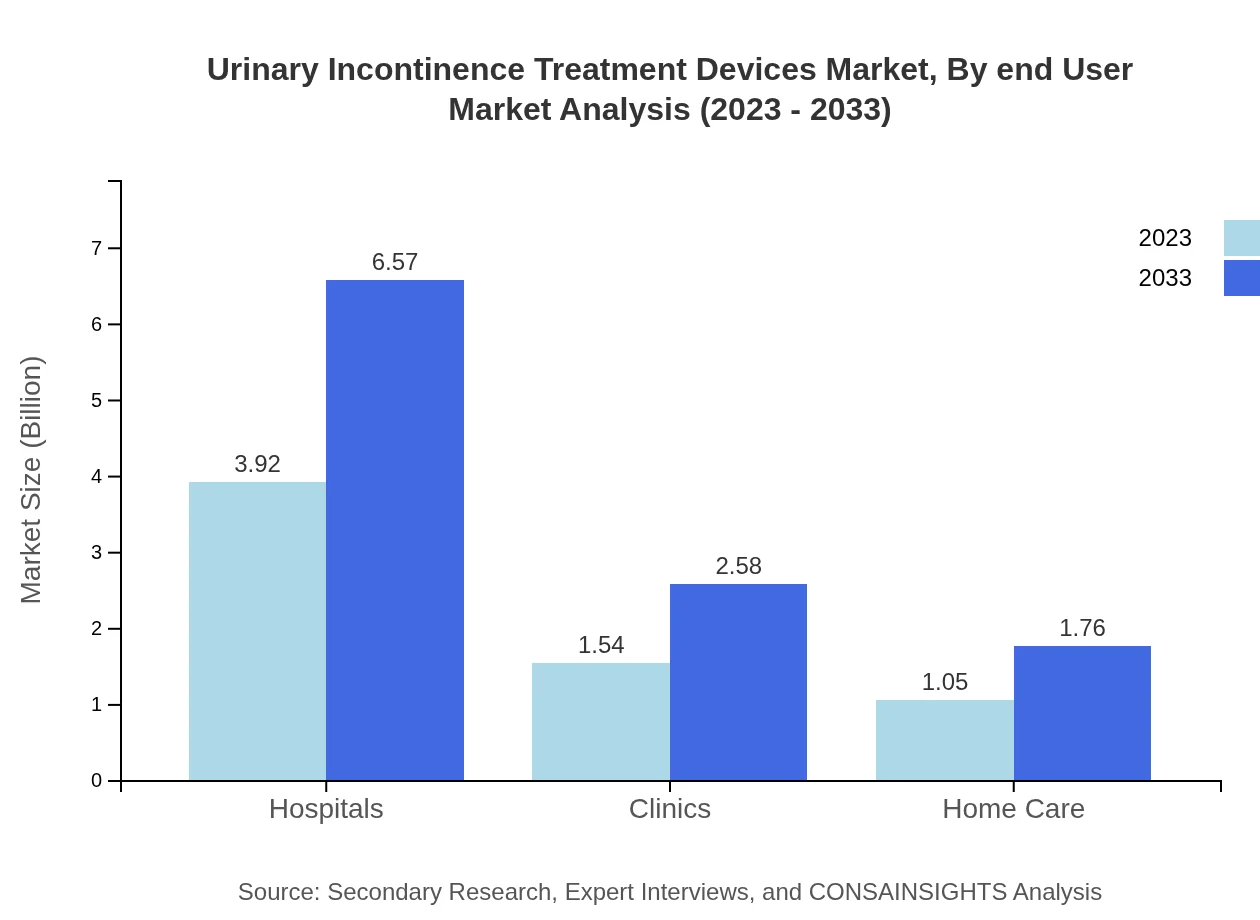
The end-user segmentation further highlights the distribution channels including hospitals, clinics, and home care settings. Hospitals held the largest market share of 60.24% in 2023, remaining consistent into 2033. Home care devices are projected to increase owing to the trend towards home-based patient management, growing from $1.05 billion in 2023 to $1.76 billion by 2033.
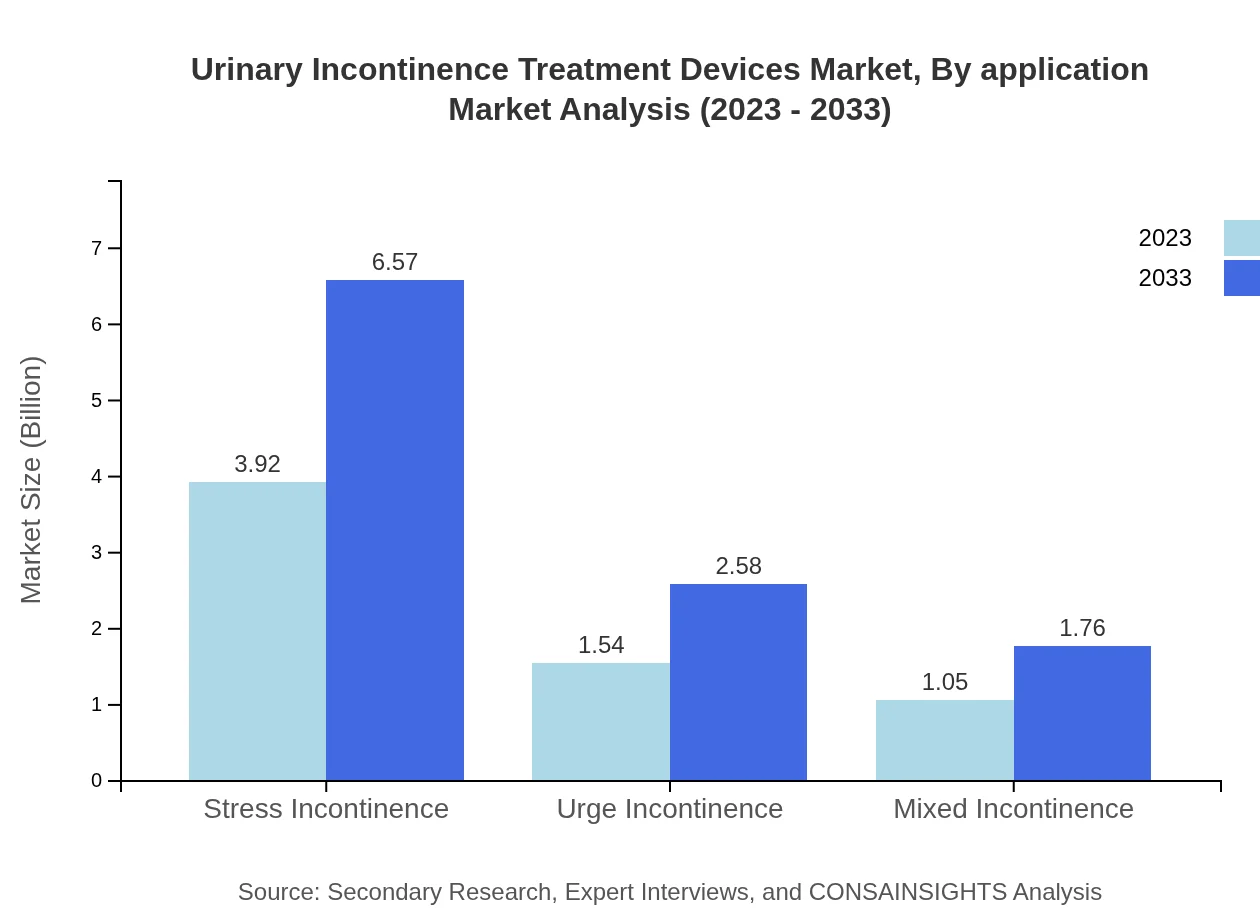
Urinary Incontinence Treatment Devices Market Analysis By Application
The application segment focuses on conditions such as stress incontinence, urge incontinence, and mixed incontinence. Stress incontinence products generated approximately $3.92 billion in 2023 and are expected to reflect similar trends into the future, maintaining substantial market shares for effective treatment solutions.
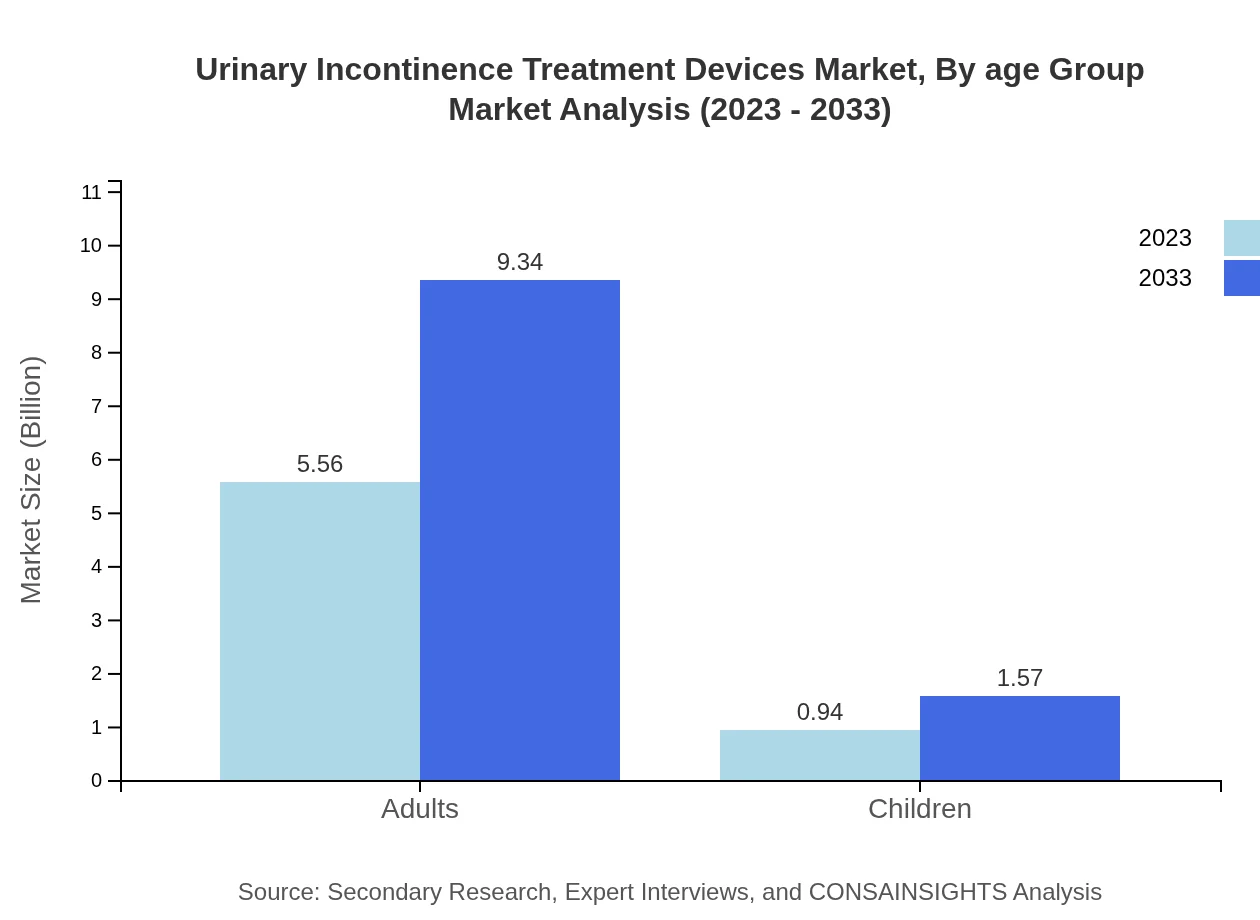
Urinary Incontinence Treatment Devices Market Analysis By Age Group
The age group segment distinguishing adults from children indicates adults dominate the market with a size of $5.56 billion in 2023, increasing to $9.34 billion, reflecting a higher prevalence in older age demographics. Children, while a smaller segment, are projected to grow gradually, reaching $1.57 billion by 2033.
Urinary Incontinence Treatment Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Urinary Incontinence Treatment Devices Industry
Medtronic :
Medtronic is a global leader in medical technology, providing diverse devices for urinary incontinence treatment and innovative solutions aimed at improving patient care.Coloplast A/S:
Coloplast specializes in medical devices for ostomy, urology, and wound care, focusing on developing solutions that enhance the quality of life for individuals with urinary incontinence.Boston Scientific Corporation:
Boston Scientific offers a wide range of products for urinary incontinence, with a commitment to innovation and effective patient outcomes, including both surgical and minimally invasive treatments.Astellas Pharma Inc.:
Astellas is dedicated to advancing healthcare options for urinary incontinence through pharmaceutical and medical device integrations, enhancing patient satisfaction.Stryker Corporation:
Stryker is renowned for its cutting-edge technology and equipment designed specifically for urinary incontinence, reflecting a strong emphasis on surgical solutions and patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of urinary Incontinence Treatment Devices?
The urinary incontinence treatment devices market is currently valued at approximately $6.5 billion in 2023, with an expected growth rate (CAGR) of 5.2%, forecasted to reach new heights by 2033.
What are the key market players or companies in this urinary Incontinence Treatment Devices industry?
Key players in the urinary incontinence treatment devices market include established companies that focus on manufacturing surgical and non-surgical treatment devices, specifically targeting innovative solutions and advanced technology to improve patient outcomes.
What are the primary factors driving the growth in the urinary incontinence treatment devices industry?
Growth in this market is driven by an aging population, increasing awareness of urinary incontinence issues, advancements in device technology, and the rising demand for effective treatment solutions to enhance quality of life for affected individuals.
Which region is the fastest Growing in the urinary Incontinence Treatment Devices?
Currently, North America is the fastest-growing region in the urinary incontinence treatment devices market, expected to rise from $2.53 billion in 2023 to $4.24 billion by 2033, driven by high healthcare expenditure and advanced healthcare infrastructure.
Does ConsaInsights provide customized market report data for the urinary Incontinence Treatment Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the urinary incontinence treatment devices industry, allowing clients to focus on particular regions, segments, or trends relevant to their business.
What deliverables can I expect from this urinary Incontinence Treatment Devices market research project?
Clients can expect comprehensive market analysis reports, including market size, growth forecasts, competitive landscape, segment analysis, trends, and strategic recommendations tailored to their specific interests in the urinary incontinence treatment devices market.
What are the market trends of urinary Incontinence Treatment Devices?
Emerging trends in the urinary incontinence treatment devices market include a shift towards less invasive treatment options, increasing investment in R&D for innovative devices, and growing telehealth applications enhancing patient access to treatments.