Biosimilar Testing And Development Services Market Report
Published Date: 31 January 2026 | Report Code: biosimilar-testing-and-development-services
Biosimilar Testing And Development Services Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the biosimilar testing and development services market, covering insights into market trends, size, segmentation, and regional dynamics from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
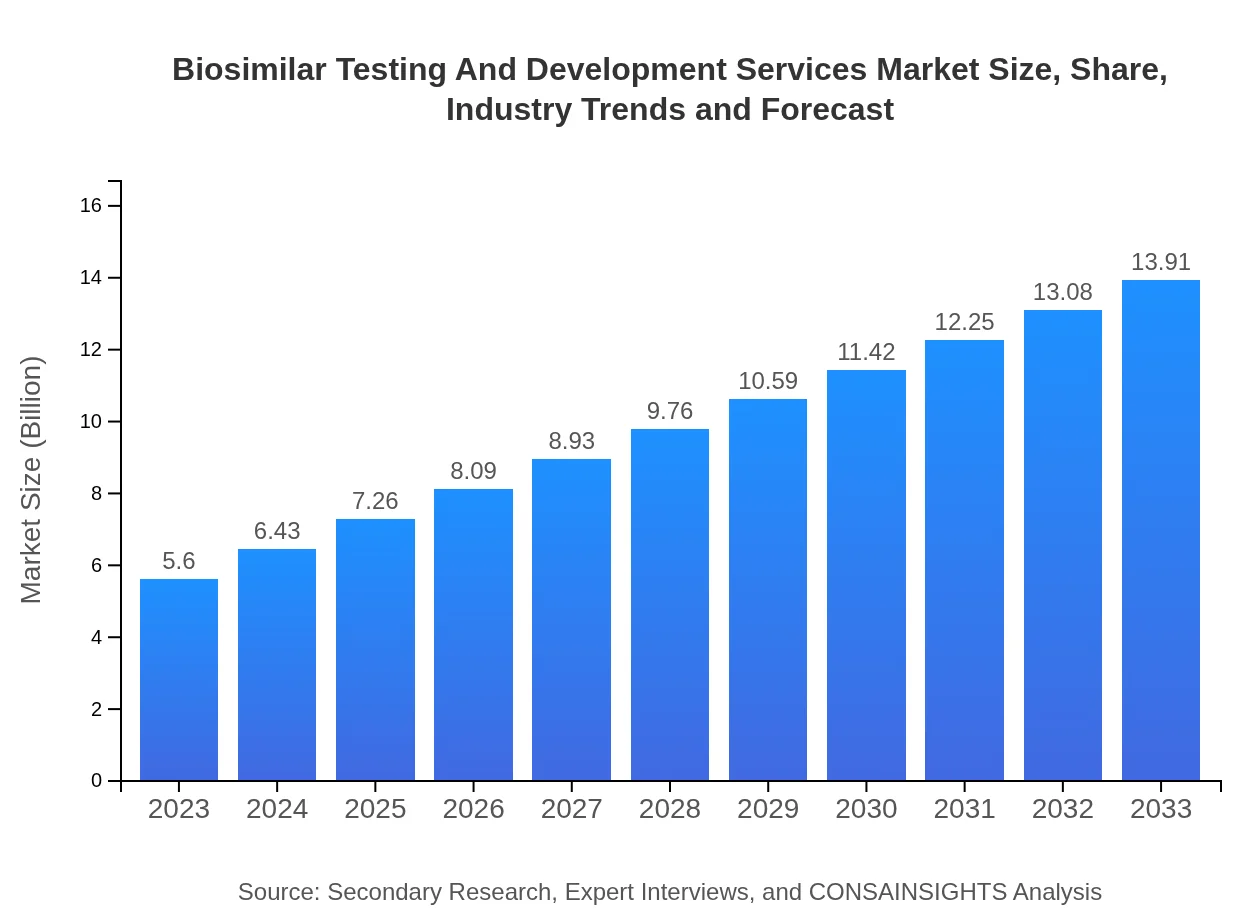
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.2% |
| 2033 Market Size | $13.91 Billion |
| Top Companies | Amgen Inc., Samsung Bioepis, Sandoz (Novartis), Pfizer Inc. |
| Last Modified Date | 31 January 2026 |
Biosimilar Testing And Development Services Market Overview
Customize Biosimilar Testing And Development Services Market Report market research report
- ✔ Get in-depth analysis of Biosimilar Testing And Development Services market size, growth, and forecasts.
- ✔ Understand Biosimilar Testing And Development Services's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biosimilar Testing And Development Services
What is the Market Size & CAGR of Biosimilar Testing And Development Services market in 2023?
Biosimilar Testing And Development Services Industry Analysis
Biosimilar Testing And Development Services Market Segmentation and Scope
Tell us your focus area and get a customized research report.
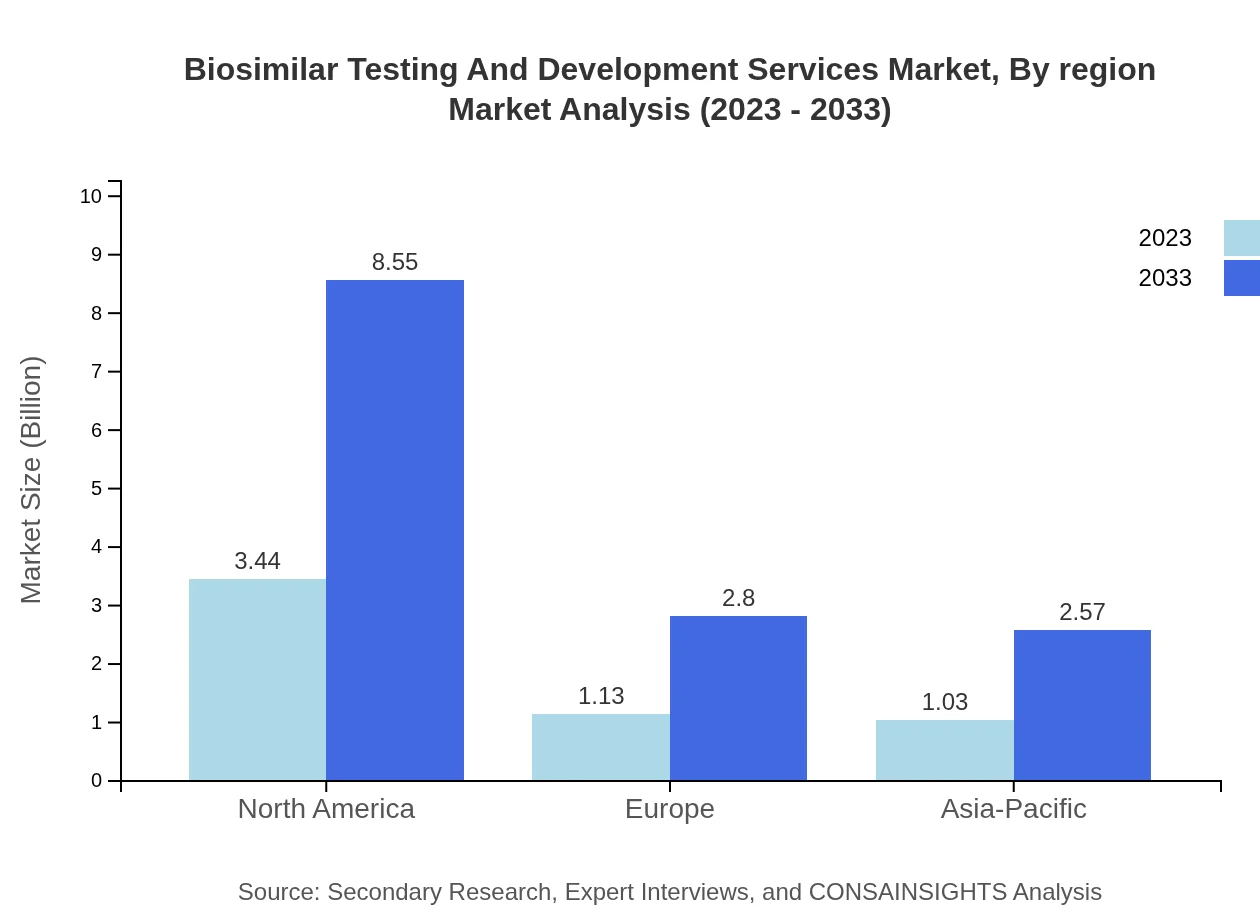
Biosimilar Testing And Development Services Market Analysis Report by Region
Europe Biosimilar Testing And Development Services Market Report:
In Europe, the biosimilar testing and development services market is anticipated to grow from $1.47 billion in 2023 to $3.66 billion by 2033. The European Medicines Agency (EMA)'s proactive approach toward biosimilars and the growing market acceptance contribute to this robust growth trajectory as companies strive to meet regulatory requirements and enhance patient access.Asia Pacific Biosimilar Testing And Development Services Market Report:
In the Asia Pacific region, the biosimilar testing and development services market is projected to grow from $1.20 billion in 2023 to $2.97 billion by 2033. The burgeoning biopharmaceutical industry, coupled with favorable government initiatives and increasing healthcare spending, positions the region as a significant growth hub. Many biopharmaceutical companies are investing in biosimilar development to leverage the growing market potential.North America Biosimilar Testing And Development Services Market Report:
North America remains a dominant player, with the market size projected to increase from $1.92 billion in 2023 to $4.78 billion by 2033. The region benefits from advanced healthcare infrastructure, significant R&D investments, and a large presence of key pharmaceutical companies driving the testing and development of biosimilars.South America Biosimilar Testing And Development Services Market Report:
The South American market for biosimilar testing and development services is expected to expand from $0.42 billion in 2023 to $1.05 billion by 2033. With increasing awareness of the benefits of biosimilars and the need for cost-effective treatment options, the region is seeing a surge in interest from biopharmaceutical companies looking to enhance their portfolios with biosimilar products.Middle East & Africa Biosimilar Testing And Development Services Market Report:
The market in the Middle East and Africa is forecasted to grow from $0.58 billion in 2023 to $1.45 billion by 2033. While the market is still emerging, there is increasing investment in healthcare infrastructure and rising demand for biosimilars as a sustainable solution to healthcare challenges in the region.Tell us your focus area and get a customized research report.
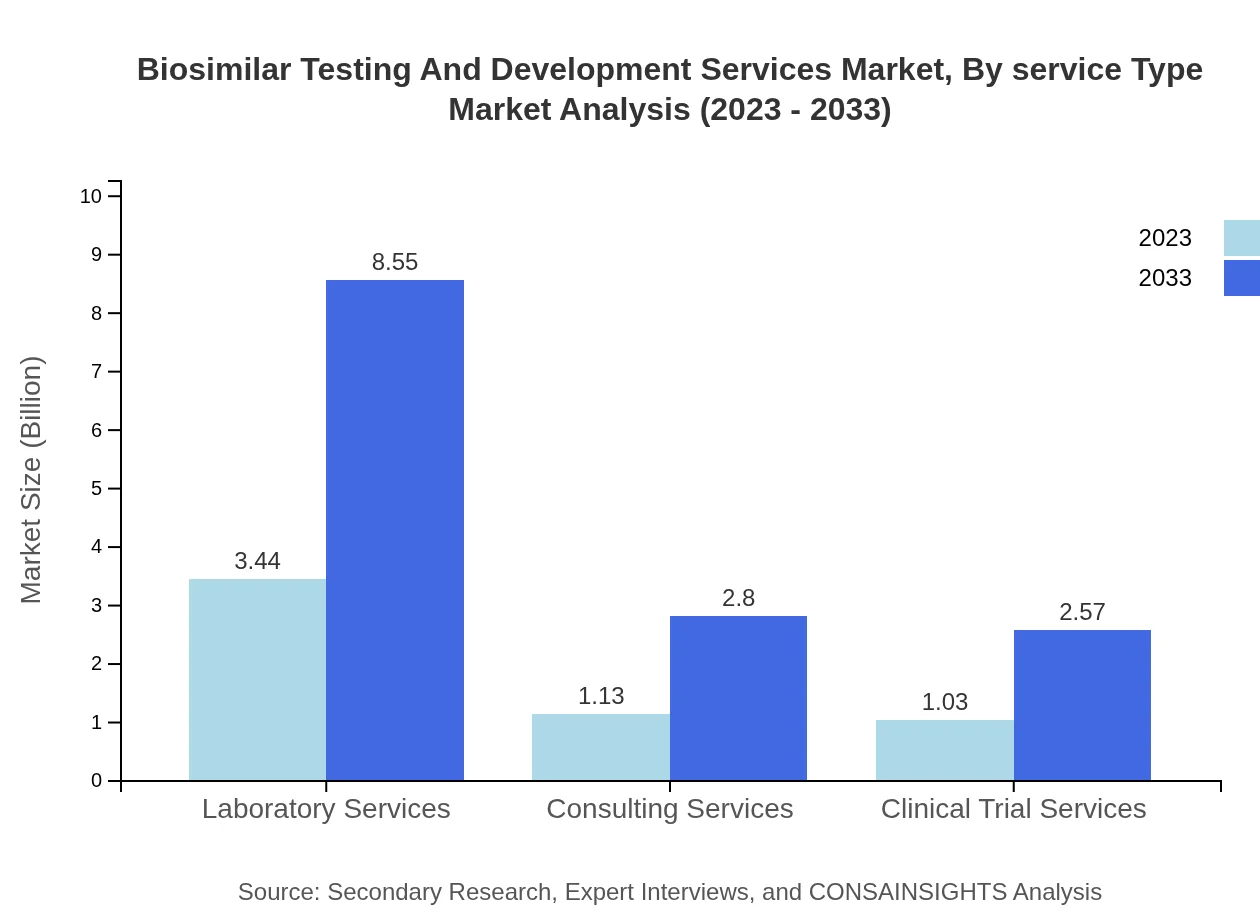
Biosimilar Testing And Development Services Market Analysis By Service Type
The service type segmentation reveals that laboratory services account for the largest share of the market, valued at $3.44 billion in 2023 and expected to reach $8.55 billion by 2033, representing 61.42% of the total market. Clinical trial services and consulting services are also significant contributors, valued at approximately $1.03 billion and $1.13 billion, respectively, in 2023.
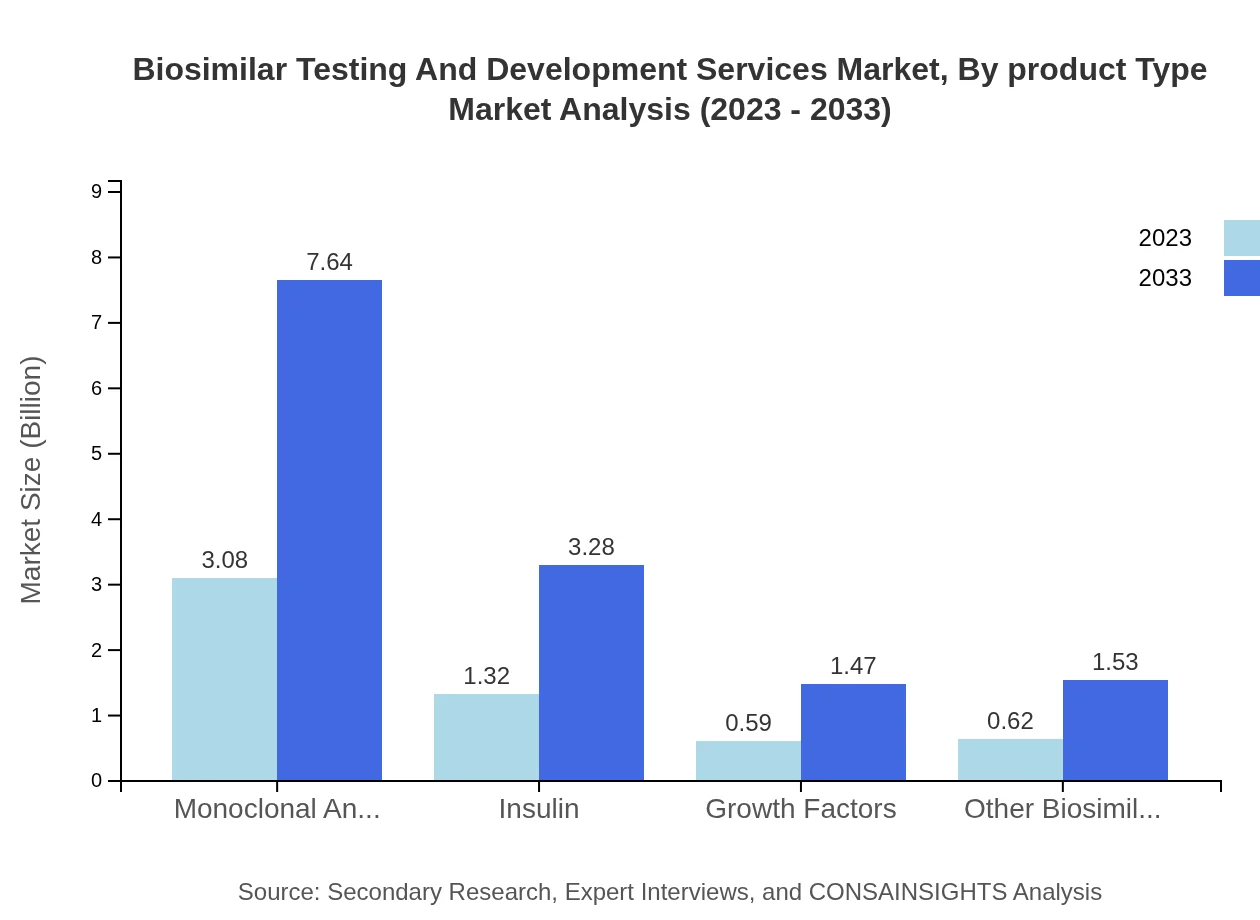
Biosimilar Testing And Development Services Market Analysis By Product Type
In terms of product types, monoclonal antibodies dominate with a market size of $3.08 billion in 2023, expected to rise significantly to $7.64 billion by 2033. Insulin and other biosimilars also represent important segments of this market, reflecting the ongoing trend towards biologic therapies.
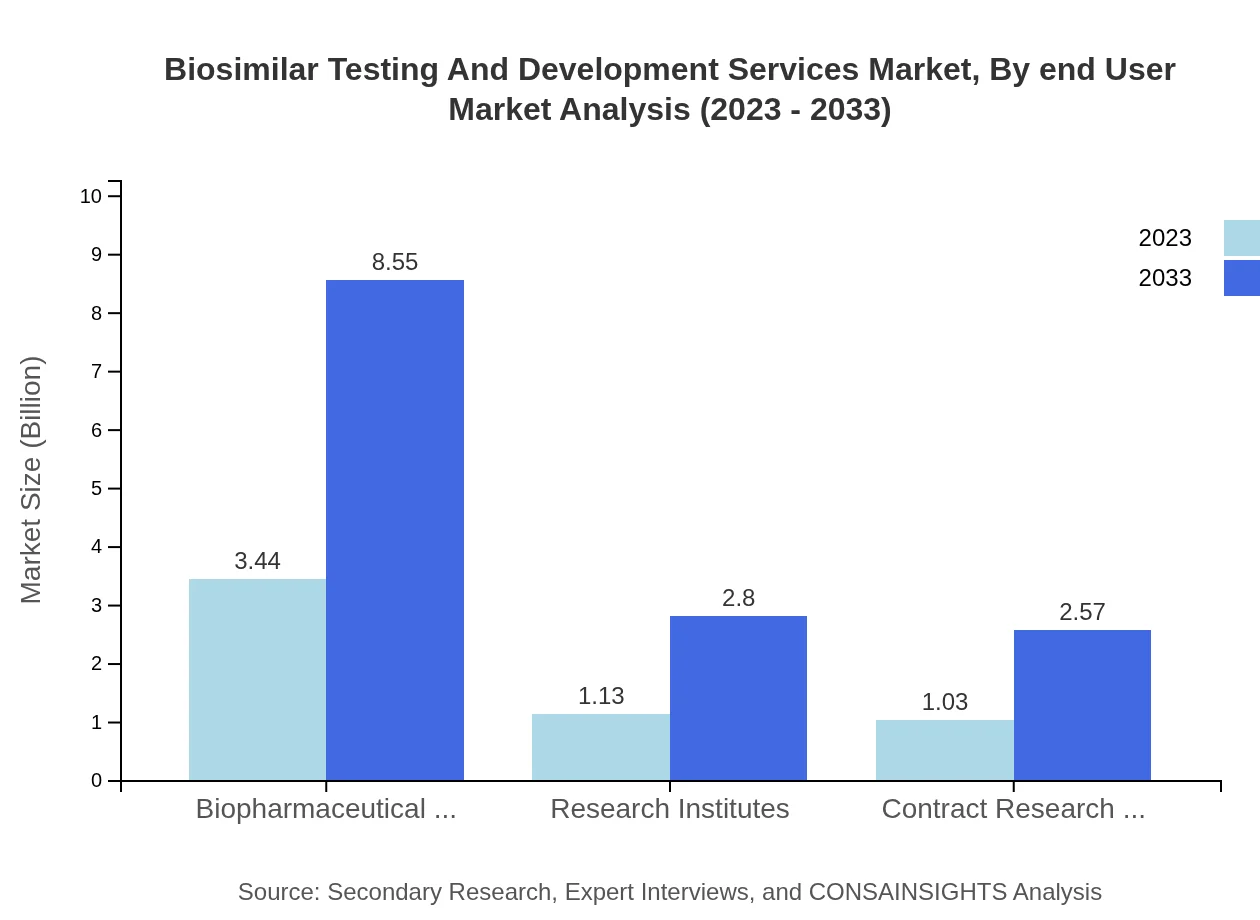
Biosimilar Testing And Development Services Market Analysis By End User
The biopharmaceutical companies segment leads the market, holding a share of 61.42% in 2023, with a size estimated at $3.44 billion. Research institutes and CROs are also key players, accounting for 20.1% and 18.48% of the market, respectively.
Biosimilar Testing And Development Services Market Analysis By Region
Regional analysis shows that North America leads in market size and growth potential, followed by Europe. The Asia Pacific region is rapidly expanding, stimulated by increasing investments in the biopharmaceutical sector. The South American and Middle East markets are also gaining traction, driven by rising healthcare needs.
Biosimilar Testing And Development Services Market Analysis By Application
Oncology remains a prominent application area for biosimilars, with a significant market portion attributed to cancer-related therapies. Autoimmune disorders and metabolic disorders are also growing applications, indicating a shifting landscape toward personalized medicine.
Biosimilar Testing And Development Services Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biosimilar Testing And Development Services Industry
Amgen Inc.:
A leader in biotechnology, Amgen focuses on developing innovative therapies, including biosimilars, contributing significantly to the overall market.Samsung Bioepis:
A major player in the biosimilars market, Samsung Bioepis specializes in wide-ranging biopharmaceutical development and offers comprehensive biosimilar services.Sandoz (Novartis):
Sandoz is dedicated to developing and manufacturing high-quality biosimilars, reinforcing their position within the global biosimilars industry.Pfizer Inc.:
With a strong portfolio of biosimilars, Pfizer is committed to delivering affordable treatment alternatives and enhancing patient access to essential medicines.We're grateful to work with incredible clients.









FAQs
What is the market size of biosimilar Testing And Development Services?
The biosimilar testing and development services market is valued at approximately $5.6 billion in 2023, with a projected annual growth rate (CAGR) of 9.2%, indicating a strong upward trend over the next decade.
What are the key market players or companies in this biosimilar Testing And Development Services industry?
Key players in the biosimilar testing and development services market include leading biopharmaceutical companies and contract research organizations (CROs), which dominate the landscape by providing extensive testing and development services for biosimilars.
What are the primary factors driving the growth in the biosimilar Testing And Development Services industry?
Key growth drivers in this industry include rising demand for affordable biopharmaceuticals, increasing healthcare expenditure, and expanding regulatory frameworks that support biosimilar development and testing, encouraging innovation and market entry.
Which region is the fastest Growing in the biosimilar Testing And Development Services?
The Asia-Pacific region is the fastest-growing market for biosimilar testing and development services, anticipated to grow from $1.20 billion in 2023 to $2.97 billion by 2033, reflecting a substantial increase in biopharmaceutical activities.
Does ConsaInsights provide customized market report data for the biosimilar Testing And Development Services industry?
Yes, ConsaInsights offers customized market reports tailored to specific client needs, providing detailed insights and comprehensive analysis in the biosimilar testing and development services industry.
What deliverables can I expect from this biosimilar Testing And Development Services market research project?
Deliverables from this market research project will include detailed market analysis, regional insights, competitive landscape overview, market trends, and future forecasts tailored to the biosimilar testing and development services sector.
What are the market trends of biosimilar Testing And Development Services?
Market trends include increasing investment in biosimilar research, accelerated regulatory approvals, rising competition among manufacturers, and a shift towards personalized medicine, influencing the dynamics of biosimilar development services.