Rsv Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: rsv-diagnostics
Rsv Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report provides comprehensive insights into the Rsv Diagnostics market, detailing current conditions and future growth forecasts from 2023 to 2033. It covers market size, segmentation, technological advancements, regional analysis, and trends shaping the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
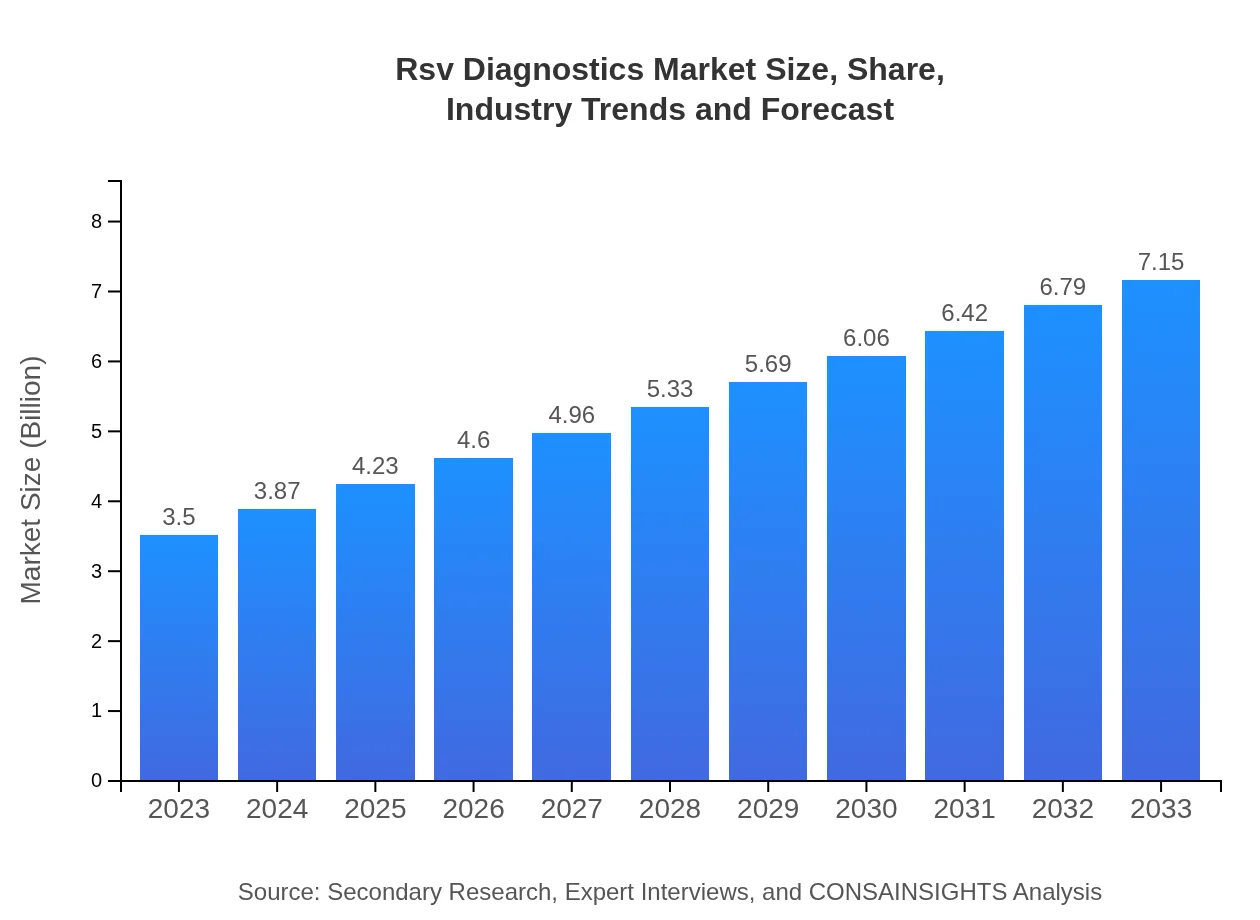
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $7.15 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Qiagen N.V., BD (Becton, Dickinson and Company), bioMerieux |
| Last Modified Date | 31 January 2026 |
Rsv Diagnostics Market Overview
Customize Rsv Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Rsv Diagnostics market size, growth, and forecasts.
- ✔ Understand Rsv Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rsv Diagnostics
What is the Market Size & CAGR of Rsv Diagnostics market in 2023?
Rsv Diagnostics Industry Analysis
Rsv Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Rsv Diagnostics Market Analysis Report by Region
Europe Rsv Diagnostics Market Report:
In Europe, the market was valued at $1.03 billion in 2023 and is projected to reach $2.10 billion by 2033. The growing elderly population and increasing healthcare expenditures drive market expansion.Asia Pacific Rsv Diagnostics Market Report:
In the Asia Pacific region, the Rsv Diagnostics market was valued at $0.68 billion in 2023 and is projected to grow to $1.39 billion by 2033. Increased incidence rates, coupled with rising awareness and healthcare investments, are driving growth.North America Rsv Diagnostics Market Report:
North America is a leading market, valued at $1.26 billion in 2023, with forecasts indicating growth to $2.57 billion by 2033. Strong healthcare infrastructure, significant research activities, and high incidences of RSV contribute to this trend.South America Rsv Diagnostics Market Report:
The South American market for Rsv Diagnostics stood at $0.15 billion in 2023 and is expected to reach $0.31 billion by 2033. Market growth is fueled by improving healthcare access and rising infectious disease awareness.Middle East & Africa Rsv Diagnostics Market Report:
The Middle East and Africa market for Rsv Diagnostics is expected to grow from $0.39 billion in 2023 to $0.79 billion by 2033. Increasing healthcare investments and awareness of RSV diagnostics are key growth determinants.Tell us your focus area and get a customized research report.
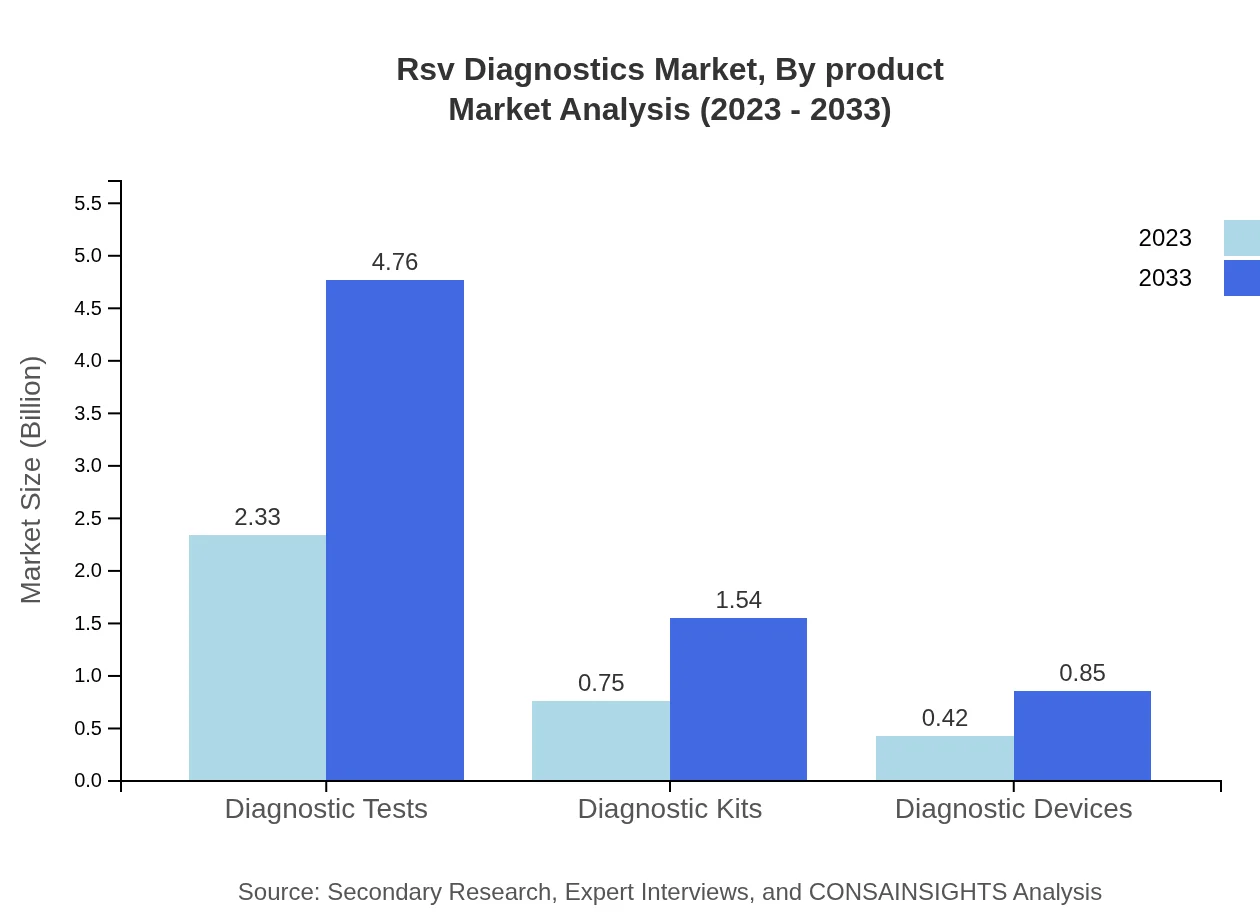
Rsv Diagnostics Market Analysis By Product
The market by product types shows that diagnostic tests are projected to grow from $2.33 billion in 2023 to $4.76 billion in 2033, capturing a significant market share of 66.49%. Diagnostic kits follow with a size of $0.75 billion in 2023, expected to reach $1.54 billion by 2033, holding a 21.57% share. Diagnostic devices currently at $0.42 billion, with predictions of $0.85 billion by 2033, account for 11.94% of the share.
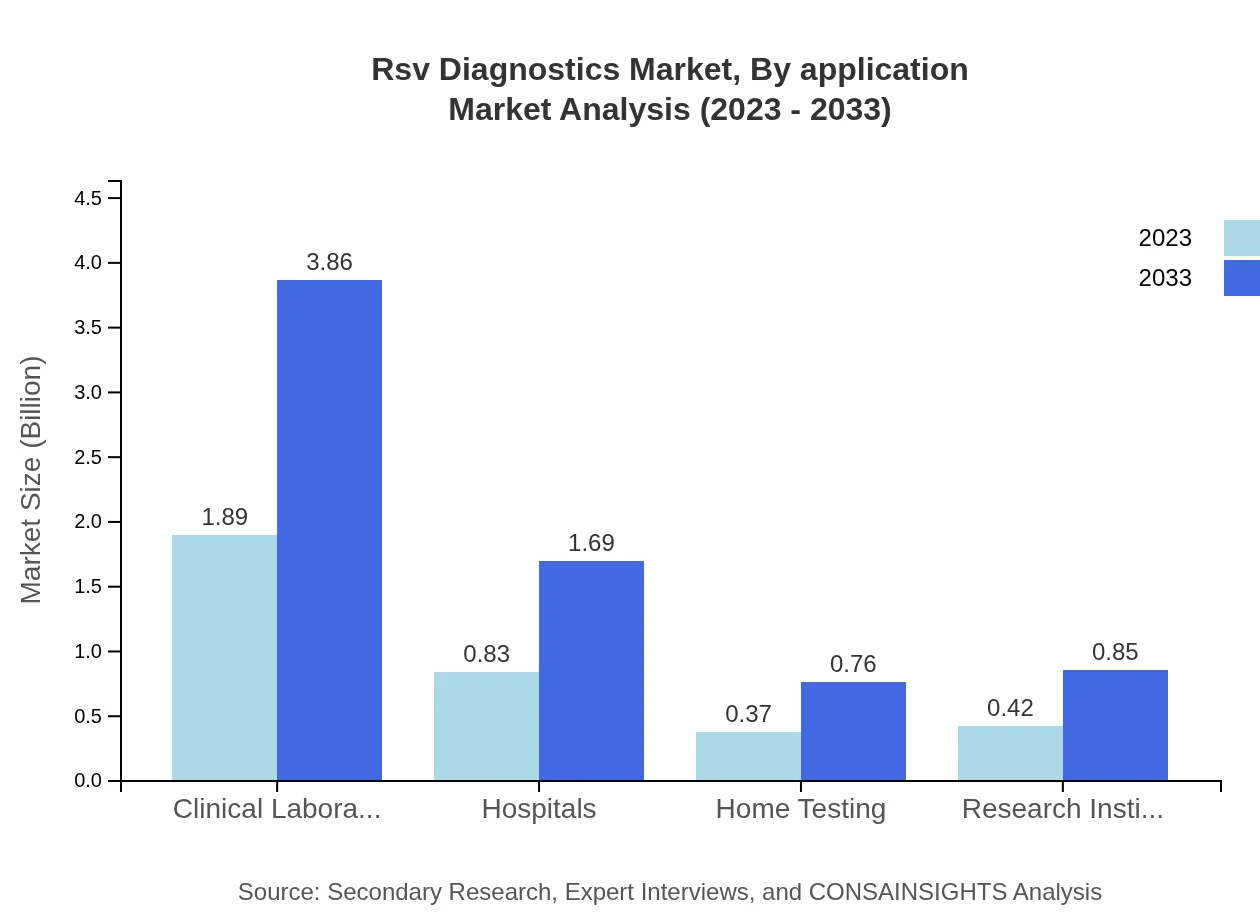
Rsv Diagnostics Market Analysis By Application
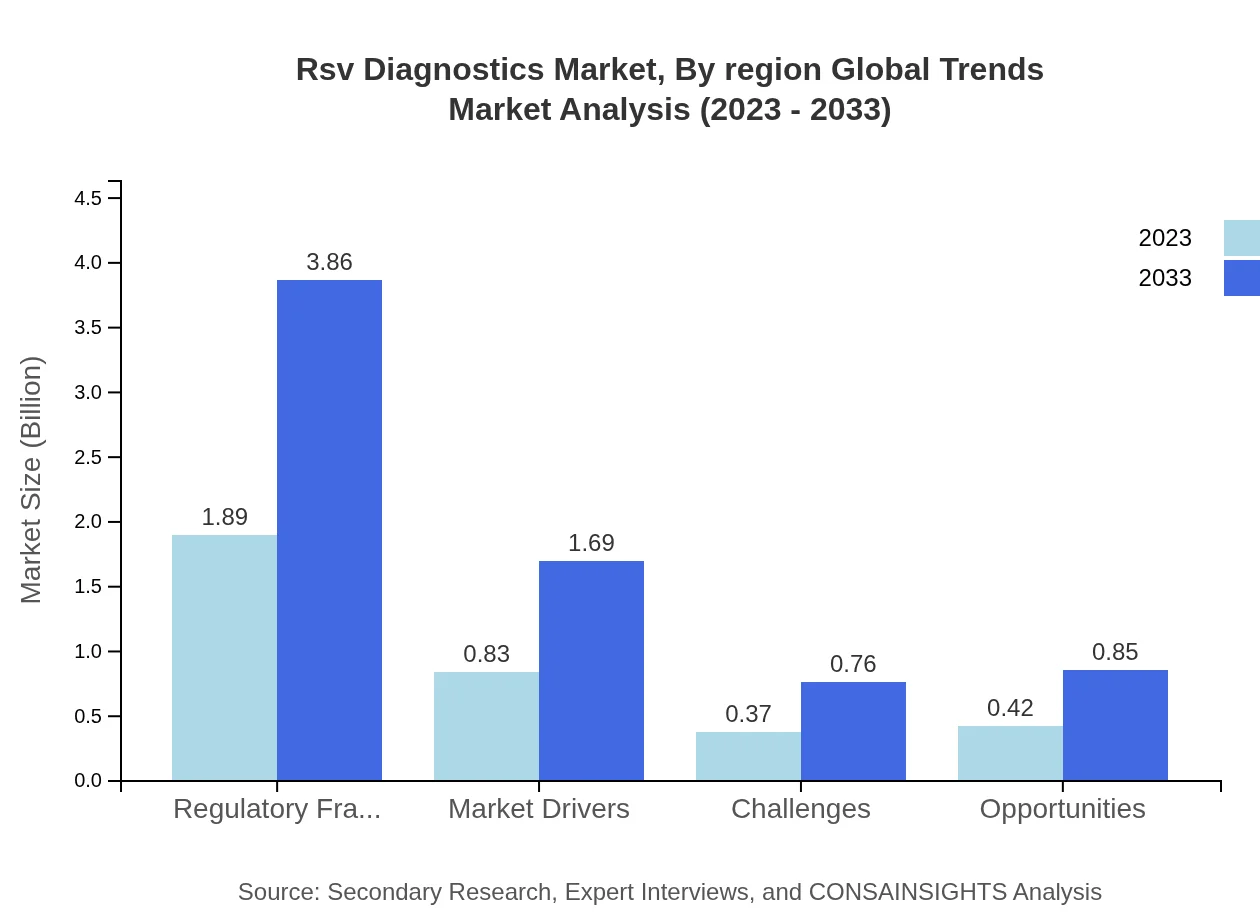
Healthcare providers, including hospitals and clinical laboratories, represent a dominant segment with $1.89 billion in 2023, growing to $3.86 billion by 2033, maintaining a significant 53.89% market share. Home testing and research institutes contribute with impressive performance, reflecting increasing demand for accessible diagnostic solutions.
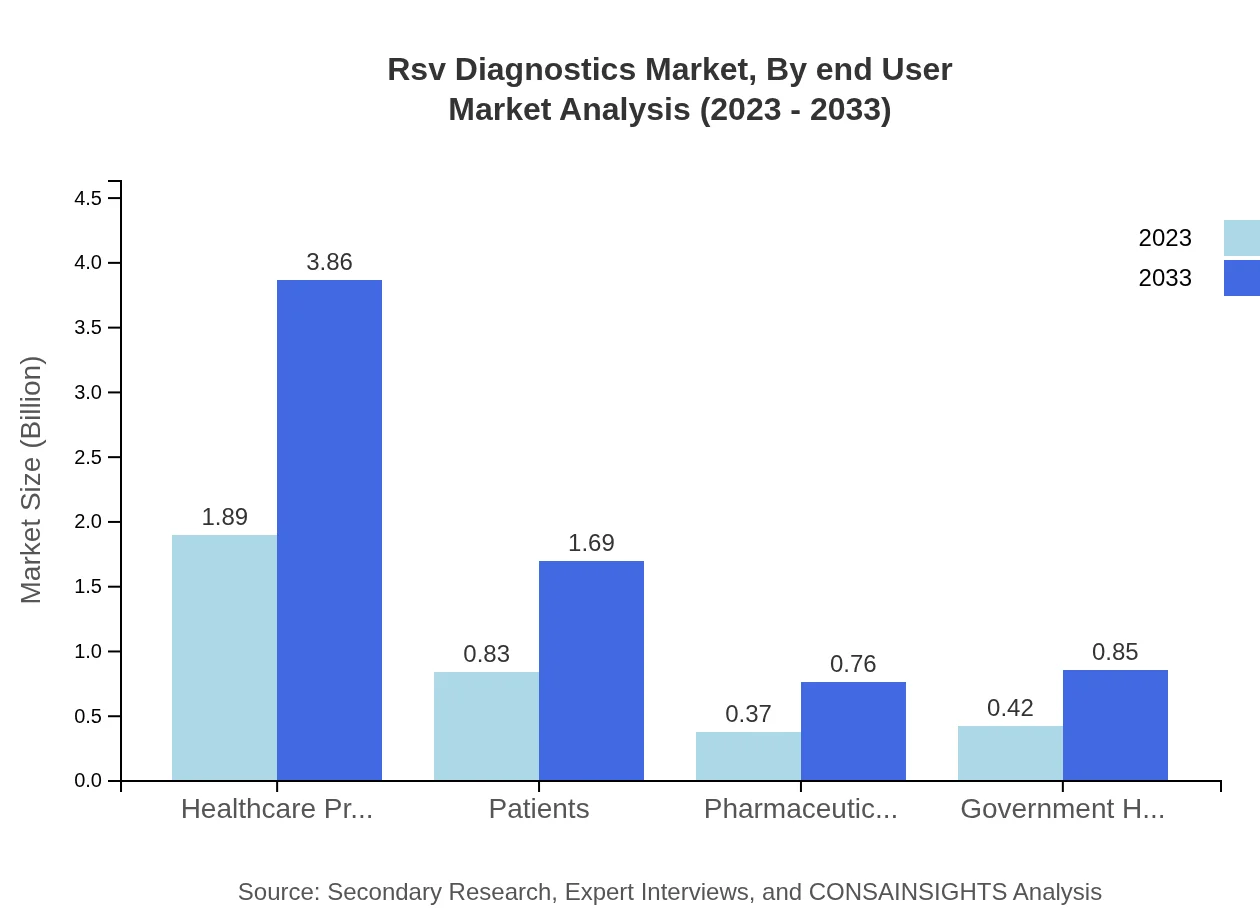
Rsv Diagnostics Market Analysis By End User
The end-user segment displays a strong focus on clinical laboratories and hospitals, each valued at $1.89 billion presently, expected to double by 2033. Government health agencies and pharmaceutical companies also play crucial roles, enhancing diagnostics availability and encouraging early RSV interventions.
Rsv Diagnostics Market Analysis By Region Global Trends
Global trends indicate a growing preference for rapid, point-of-care diagnostics. Technological advancements, such as AI integration and telemedicine, are enhancing diagnostic capabilities and patient management, further shaping the Rsv Diagnostics market landscape.
Rsv Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rsv Diagnostics Industry
Roche Diagnostics:
Leading in molecular diagnostics, Roche provides innovative solutions for early RSV detection, emphasizing accuracy and reliability.Abbott Laboratories:
Known for its rapid diagnostic tools, Abbott is a key player focusing on enhancing the speed and efficiency of RSV diagnostics globally.Qiagen N.V.:
Pioneering in sample prep and molecular assays, Qiagen contributes significantly to the RSV diagnostics landscape with their advanced logistics.BD (Becton, Dickinson and Company):
BD offers a comprehensive portfolio for RSV diagnostics and is committed to delivering innovative diagnostic solutions.bioMerieux:
With a robust range of diagnostic products, bioMerieux specializes in aiding healthcare providers in RSV detection and management.We're grateful to work with incredible clients.









FAQs
What is the market size of rsv Diagnostics?
The RSV diagnostics market is valued at approximately $3.5 billion in 2023, with a robust compound annual growth rate (CAGR) of 7.2%. This growth trajectory is expected to enhance the market's value significantly by 2033, indicating a strong demand for these diagnostic solutions.
What are the key market players or companies in the rsv Diagnostics industry?
The RSV diagnostics industry features notable players including major pharmaceutical firms, healthcare providers, and diagnostic manufacturers. These entities drive innovation and provide advanced diagnostic kits and solutions essential for RSV detection across various healthcare settings.
What are the primary factors driving the growth in the rsv Diagnostics industry?
Key growth drivers for the RSV diagnostics market include increasing RSV infection rates, advancements in diagnostic technologies, and heightened awareness among healthcare providers. Furthermore, the rise in healthcare expenditure contributes to the market's expansion as more resources are allocated for diagnostics.
Which region is the fastest Growing in the rsv Diagnostics?
The Asia Pacific region is rapidly emerging as the fastest-growing market in RSV diagnostics, expected to expand from $0.68 billion in 2023 to $1.39 billion by 2033. This growth is fueled by increasing healthcare access and rising disease incidence rates.
Does ConsaInsights provide customized market report data for the rsv Diagnostics industry?
Yes, Consainsights offers customized market report data for the RSV diagnostics industry. Clients can obtain tailored insights based on specific market segments, geographic regions, and other parameters, allowing for strategic decision-making and investment planning.
What deliverables can I expect from this rsv Diagnostics market research project?
Deliverables from the RSV diagnostics market research project typically include comprehensive market analysis reports, segmented data insights, growth forecasts, competitor analysis, and actionable recommendations. Additionally, visual aids like graphs and charts will support the findings.
What are the market trends of rsv Diagnostics?
Current trends in the RSV diagnostics market include increased demand for rapid testing solutions, integration of digital health technologies, and a focus on preventive care. Additionally, regulatory advancements and partnerships among key players are shaping the future landscape of this industry.