Viral Vector And Plasmid Dna Manufacturing Market Report
Published Date: 31 January 2026 | Report Code: viral-vector-and-plasmid-dna-manufacturing
Viral Vector And Plasmid Dna Manufacturing Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Viral Vector and Plasmid DNA Manufacturing market for the forecast period 2023-2033. It covers market size, growth trends, segmentation, and key industry leaders, offering valuable insights for stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
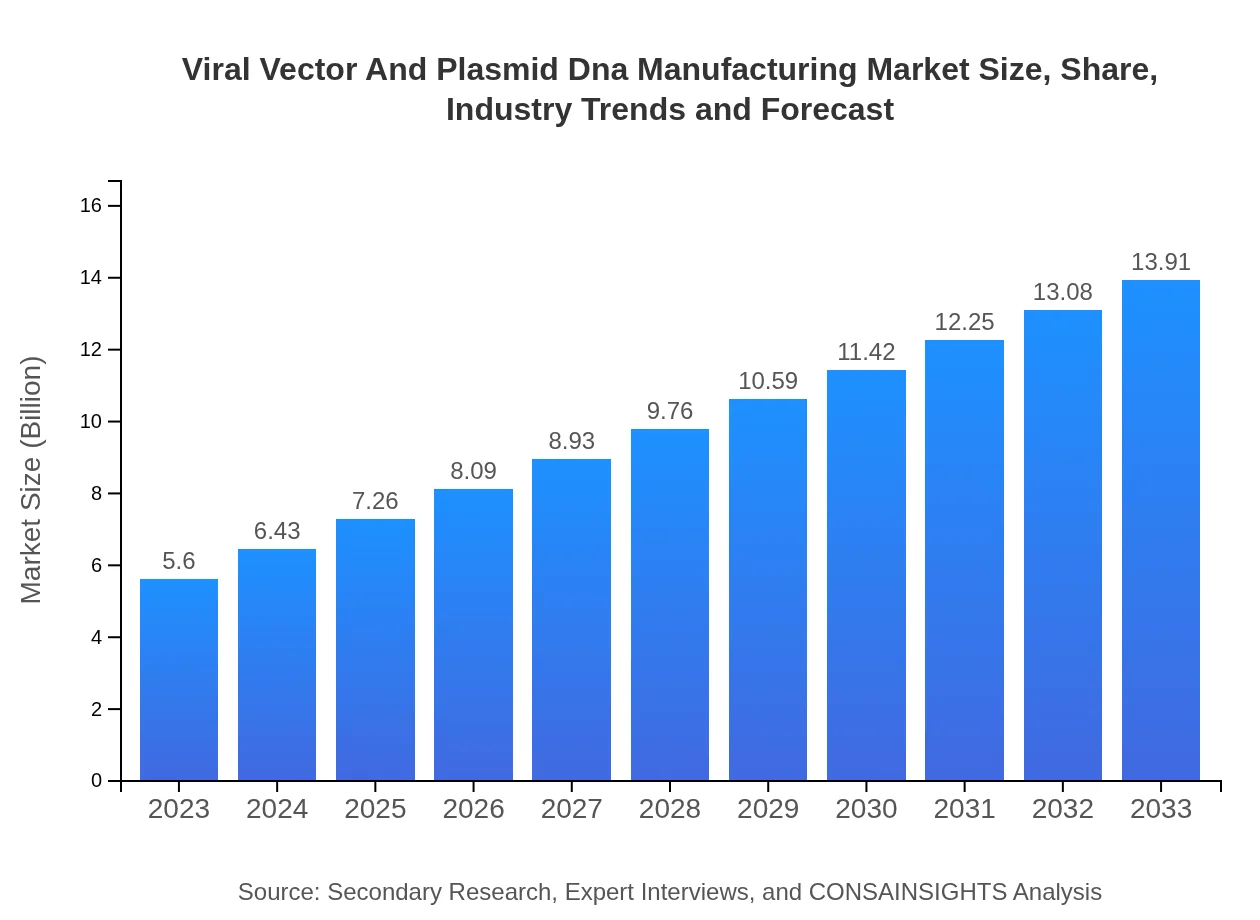
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.2% |
| 2033 Market Size | $13.91 Billion |
| Top Companies | Novartis, Lonza Group, GW Pharmaceuticals, Roche, Sangamo Therapeutics |
| Last Modified Date | 31 January 2026 |
Viral Vector And Plasmid Dna Manufacturing Market Overview
Customize Viral Vector And Plasmid Dna Manufacturing Market Report market research report
- ✔ Get in-depth analysis of Viral Vector And Plasmid Dna Manufacturing market size, growth, and forecasts.
- ✔ Understand Viral Vector And Plasmid Dna Manufacturing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Viral Vector And Plasmid Dna Manufacturing
What is the Market Size & CAGR of the Viral Vector And Plasmid Dna Manufacturing market in 2023?
Viral Vector And Plasmid Dna Manufacturing Industry Analysis
Viral Vector And Plasmid Dna Manufacturing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Viral Vector And Plasmid Dna Manufacturing Market Analysis Report by Region
Europe Viral Vector And Plasmid Dna Manufacturing Market Report:
In Europe, the market is expected to grow from USD 2.06 billion in 2023 to USD 5.12 billion in 2033. European countries are focusing heavily on personalized medicine and gene therapy, providing a conducive environment for industry growth, driven by successful clinical results and strategic funding.Asia Pacific Viral Vector And Plasmid Dna Manufacturing Market Report:
In the Asia Pacific region, the market is expected to grow from USD 1.01 billion in 2023 to USD 2.52 billion in 2033. This growth is driven by increasing investments in biotechnology and a rapidly developing healthcare infrastructure, particularly in countries like China and India, which are becoming key players in drug development and manufacturing.North America Viral Vector And Plasmid Dna Manufacturing Market Report:
North America remains a dominant region in the market, with the market size anticipated to rise from USD 1.82 billion in 2023 to USD 4.52 billion in 2033. This increase is encouraged by strong R&D investments, technological advancements, and a robust regulatory framework that supports fast-tracked approvals for viral vector products.South America Viral Vector And Plasmid Dna Manufacturing Market Report:
The South American market, though smaller, is projected to progress from USD 0.22 billion in 2023 to USD 0.54 billion in 2033. This growth is primarily attributed to rising interest in clinical trials and biopharmaceutical developments spurred by government initiatives and global partnerships.Middle East & Africa Viral Vector And Plasmid Dna Manufacturing Market Report:
The Middle East and Africa market is projected to expand from USD 0.49 billion in 2023 to USD 1.21 billion by 2033. Growth here is linked with increasing healthcare investments and advancements in cancer therapy and genetic disorders, despite challenges in regulatory environments.Tell us your focus area and get a customized research report.
Viral Vector And Plasmid Dna Manufacturing Market Analysis By Product Type
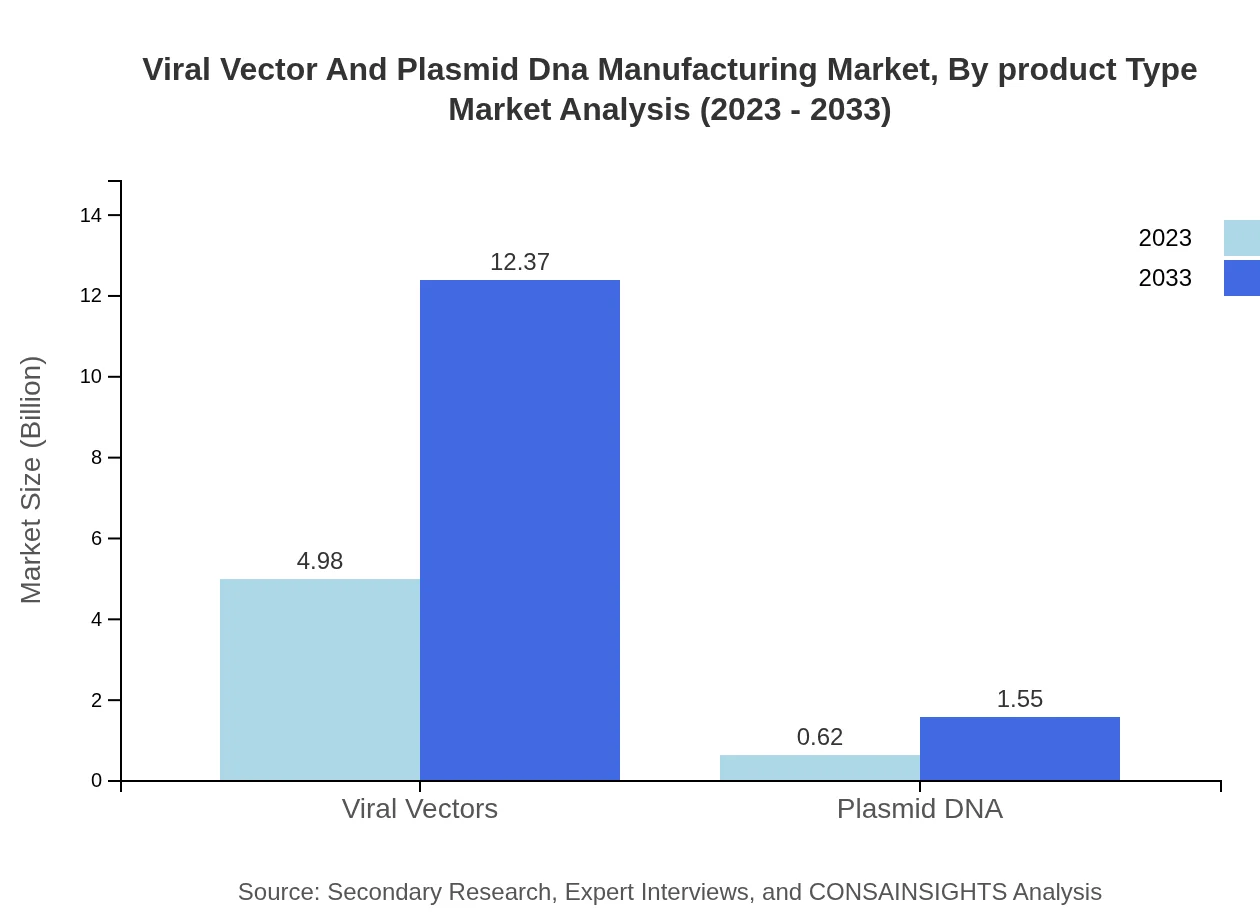
In 2023, the Viral Vectors segment dominates the market with a value of USD 4.98 billion and is projected to grow to USD 12.37 billion by 2033, capturing 88.88% share. The Plasmid DNA segment, in contrast, holds a much smaller portion at USD 0.62 billion, expanding to USD 1.55 billion, translating to an 11.12% share.
Viral Vector And Plasmid Dna Manufacturing Market Analysis By Application
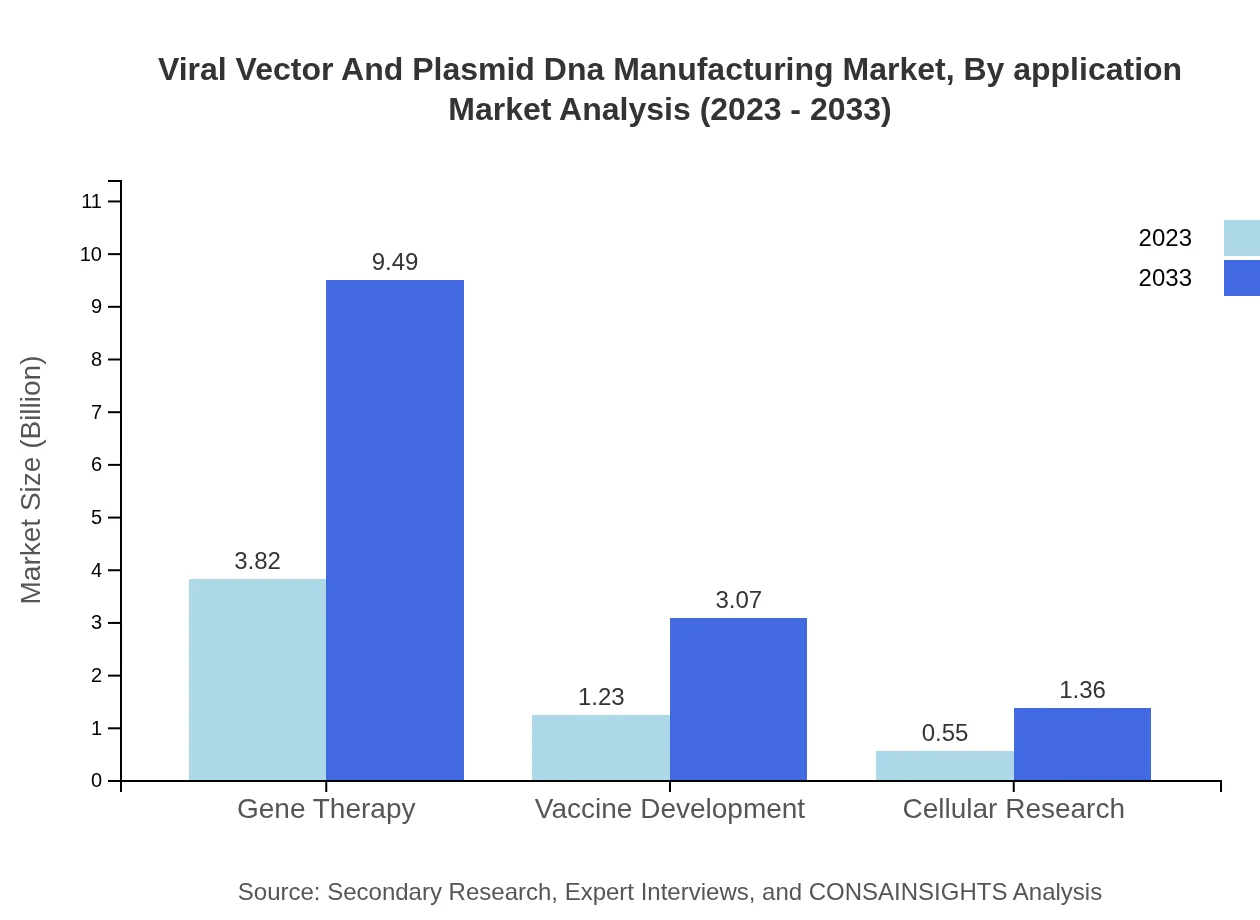
The market is segmented by application into Gene Therapy, Vaccine Development, and Cellular Research. Gene Therapy leads the market with a share of 68.23%, projected to rise from USD 3.82 billion in 2023 to USD 9.49 billion by 2033. Vaccine Development and Cellular Research represent 22.03% and 9.74% segments respectively, growing steadily in value and share.
Viral Vector And Plasmid Dna Manufacturing Market Analysis By End User
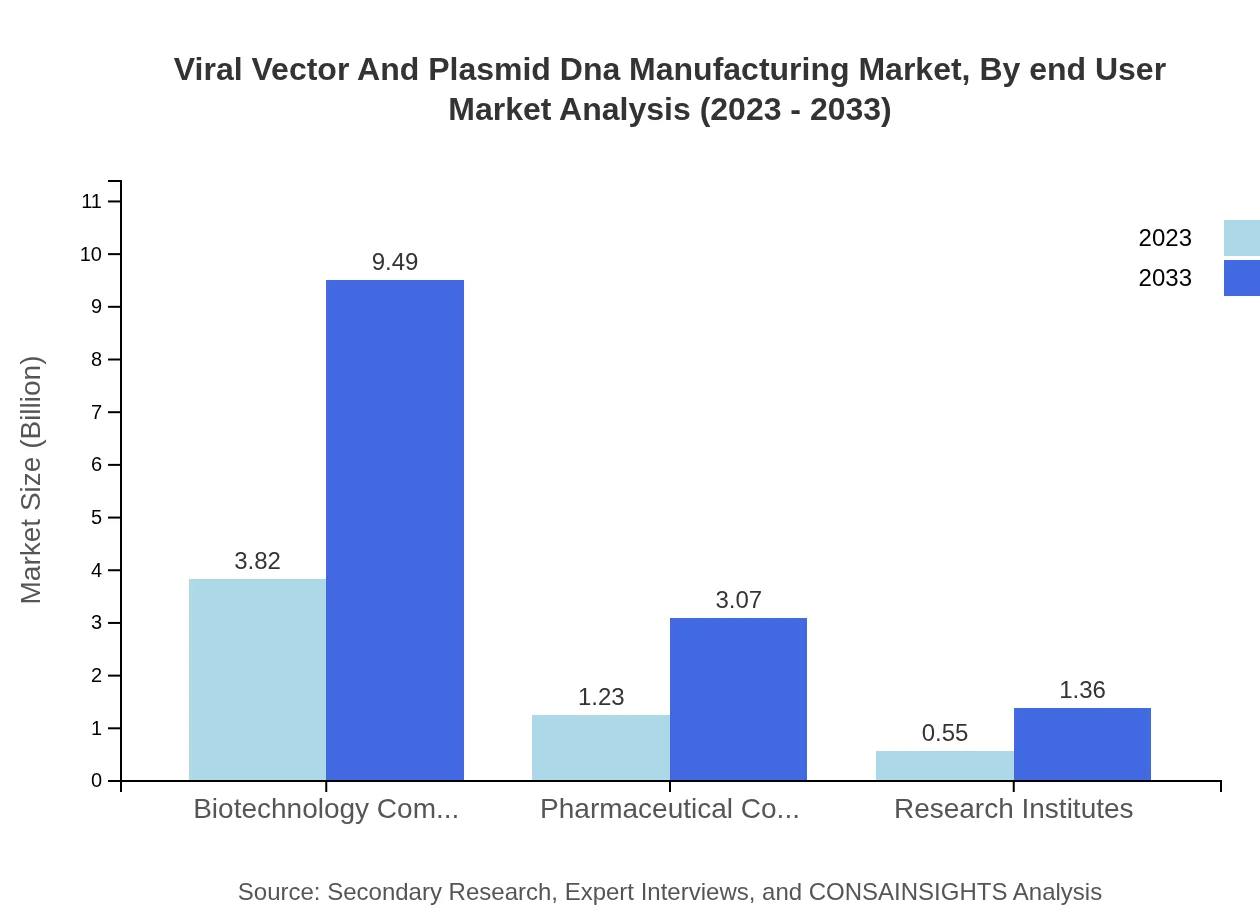
The key stakeholders in this market include Biotechnology Companies, Pharmaceutical Companies, and Research Institutes. Biotechnology Companies occupy the largest share at 68.23% (USD 3.82 billion to USD 9.49 billion), followed by Pharmaceutical Companies at 22.03% (from USD 1.23 billion to USD 3.07 billion) and Research Institutes at 9.74% (from USD 0.55 billion to USD 1.36 billion).
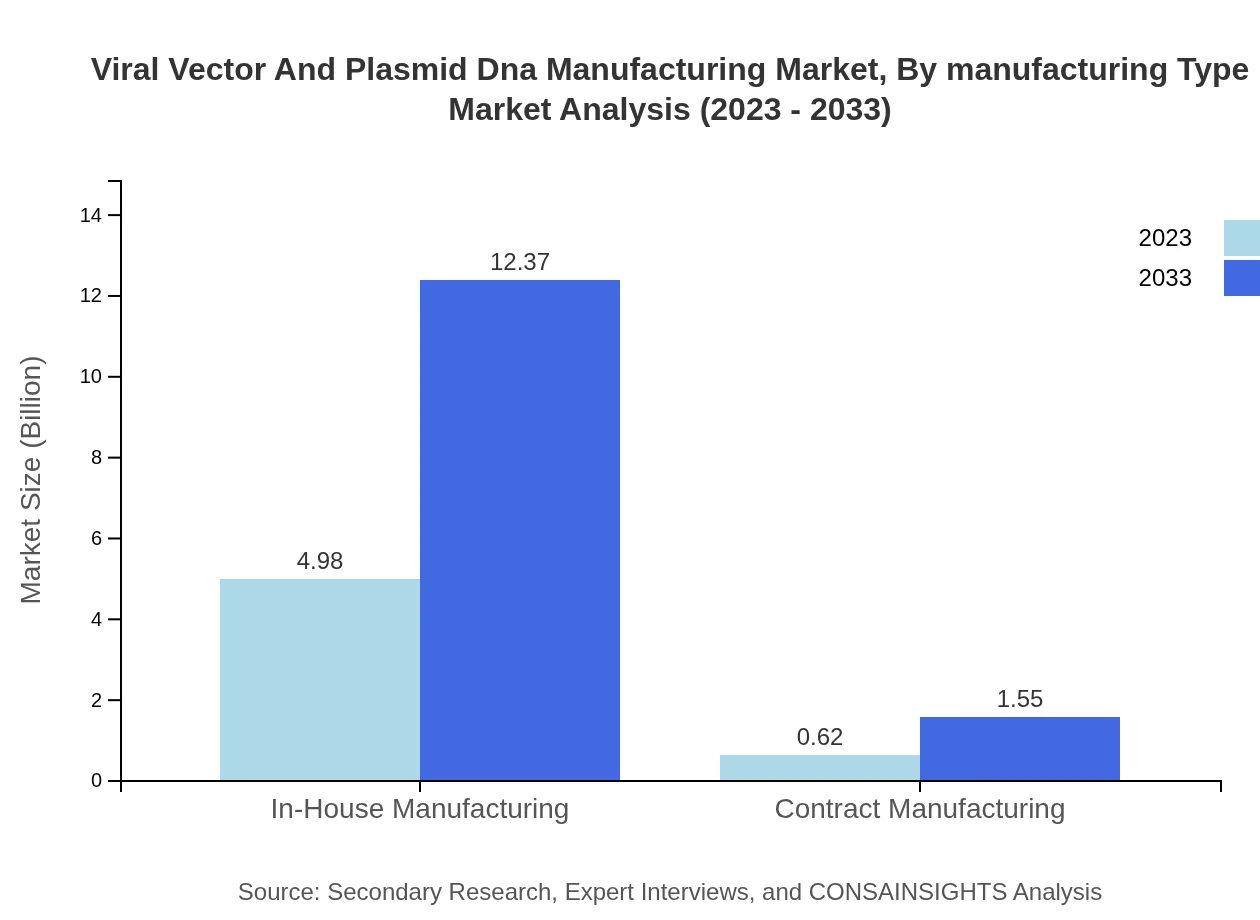
Viral Vector And Plasmid Dna Manufacturing Market Analysis By Manufacturing Type
The manufacturing segment comprises In-House Manufacturing and Contract Manufacturing. In-House Manufacturing accounts for 88.88% of the market share, moving from USD 4.98 billion in 2023 to USD 12.37 billion in 2033, while Contract Manufacturing captures 11.12%, evolving from USD 0.62 billion to USD 1.55 billion.
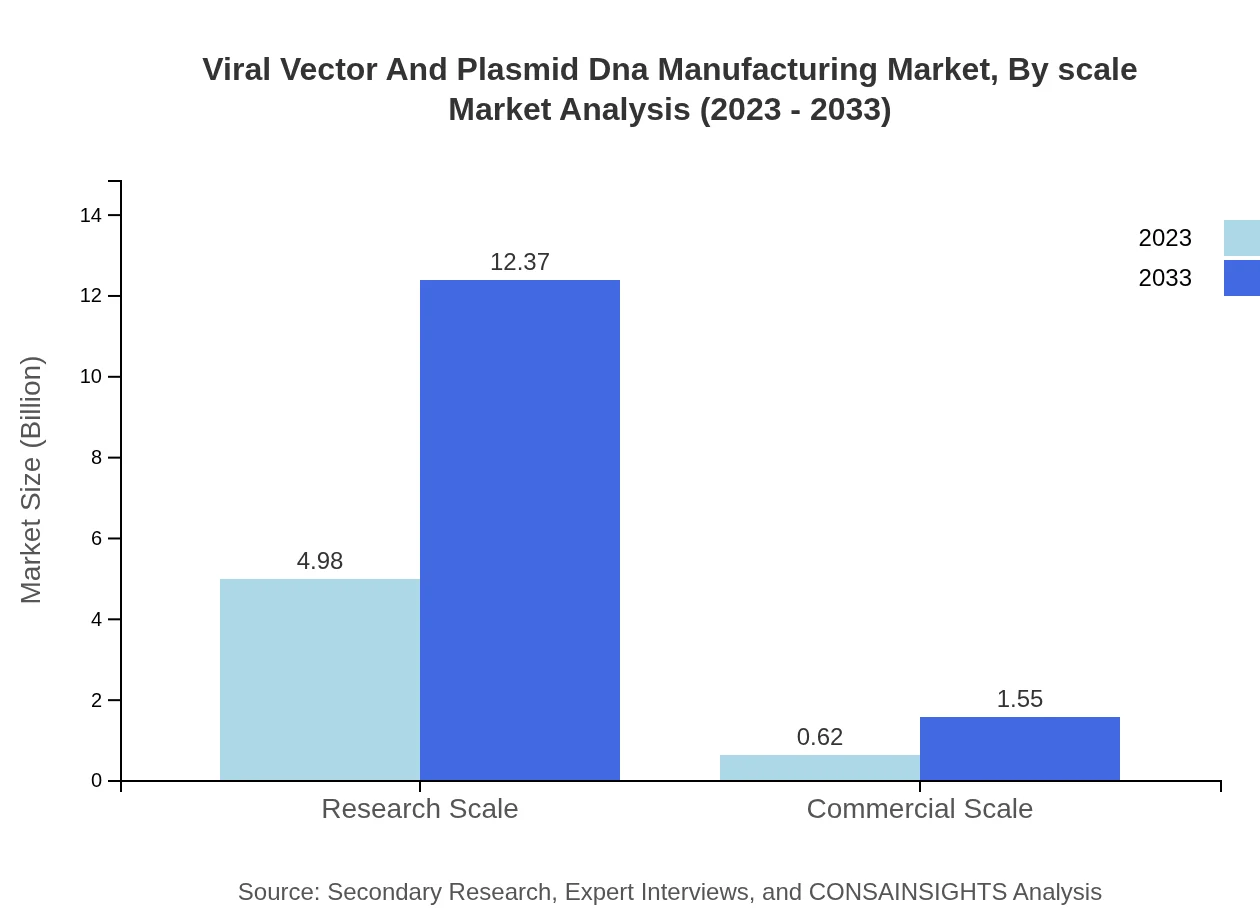
Viral Vector And Plasmid Dna Manufacturing Market Analysis By Scale
The market is distinguished by scale, with Research Scale leading with a size of USD 4.98 billion in 2023, predicted to increase to USD 12.37 billion by 2033 with a 88.88% market share. Contrarily, the Commercial Scale segment will escalate from USD 0.62 billion to USD 1.55 billion, capturing an 11.12% share.
Viral Vector And Plasmid Dna Manufacturing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in the Viral Vector And Plasmid Dna Manufacturing Industry
Novartis:
Novartis is a global healthcare company based in Switzerland that focuses on innovative medicines. They actively invest in gene therapy programs and possess robust manufacturing capabilities for viral vectors.Lonza Group:
Lonza is a leading integrated solutions provider for the pharmaceutical, biotech, and specialty ingredients markets. They offer comprehensive services in viral vector production and have established a prominent position in the CMOs sector.GW Pharmaceuticals:
GW Pharmaceuticals specializes in discovering, developing, and commercializing cannabinoid-based medicines and utilizes viral vectors to enhance treatment approaches in genetically influenced diseases.Roche:
Roche is a pioneering research-focused healthcare company that has developed comprehensive R&D programs, including platforms that utilize plasmid DNA for advanced therapies.Sangamo Therapeutics:
Sangamo Therapeutics focuses on genomic therapies to treat rare diseases. The company engages extensively in viral vector research and manufacturing, sustaining a competitive edge in the therapeutic landscape.We're grateful to work with incredible clients.









FAQs
What is the market size of viral vector and plasmid DNA manufacturing?
The viral vector and plasmid DNA manufacturing market is valued at $5.6 billion in 2023, with a projected CAGR of 9.2% from 2023 to 2033. This growth reflects increasing demand in biopharmaceuticals and genetic therapies.
What are the key market players or companies in this industry?
The key players in the viral vector and plasmid DNA manufacturing industry include major biotechnology firms and pharmaceutical companies that engage in gene therapy and vaccine development, enhancing competitive dynamics and innovation.
What are the primary factors driving the growth in the industry?
Growth in the viral vector and plasmid DNA manufacturing industry is largely driven by advancements in genetic therapies, increasing prevalence of genetic disorders, the expansion of vaccine development and rising investment in biotechnology research.
Which region is the fastest Growing in the industry?
Among various regions, Europe is projected to be the fastest-growing area in the viral vector and plasmid DNA manufacturing market, expanding from $2.06 billion in 2023 to $5.12 billion by 2033, indicating robust development.
Does ConsaInsights provide customized market report data for the industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the viral vector and plasmid DNA manufacturing industry, ensuring clients receive relevant and actionable insights for strategic decision-making.
What deliverables can I expect from this market research project?
Expect comprehensive deliverables that include market size estimates, growth forecasts, trend analyses, competitive landscape evaluations, and segmentation insights for the viral vector and plasmid DNA manufacturing market.
What are the market trends of viral vector and plasmid DNA manufacturing?
Current market trends reveal an increasing focus on personalized medicine, growth in cell and gene therapies, continuous innovation in manufacturing processes, and growing partnerships between biotech firms for advanced therapeutic solutions.